Abstract
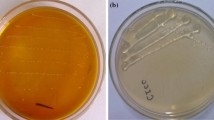
Dextrans are exo-polysaccharides prominently used as biomaterial on the grounds of their biodegradability and biocompatibility characteristics. There is an increasing interest and need to identify alternative cost-effective and renewable carbon source for dextran to reduce the production cost and to improve the overall economy of dextran production. In this work, dextran was produced using Saccharum officinarum juice (SOJ) as a low-carbon source by Leuconostoc mesenteroides MTCC 7337. The suitable condition/parameter for the production of dextran was found to be medium pH, 7; shaking speed, 150 rpm; inoculum size, 5% v/v; and nitrogen source, yeast extract. The different downstream factors including screening of solvent, supernatant to solvent ratio and precipitation time on the recovery of dextran, were studied. The organic solvent, ethanol with volume ratio of 1:4 (supernatant to solvent ratio), and precipitation time of 16 h were selected based on the maximum recovery of dextran from SOJ medium. The purified dextran was characterized by proton nuclear magnetic resonance spectroscopy (1H-NMR), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA) analysis. The results of rheological studies indicate that dextran solution behaves like a pseudoplastic fluid at higher concentrations of dextran solution. Based on the results, it was found that SOJ could be used as an alternate substrate for the production of dextran.








Similar content being viewed by others
References
Ponnusami V, Gunasekar V (2015) Production of pullulan by microbial fermentation. In: Polysaccharides: Bioactivity and Biotechnology, pp 1–2241
Li RH, Bin ZH, Hu XQ et al (2016) An efficiently sustainable dextran-based flocculant: synthesis, characterization and flocculation. Chemosphere 159:342–350
Capek P, Hlavoňová E, Matulová M et al (2011) Isolation and characterization of an extracellular glucan produced by Leuconostoc garlicum PR. Carbohydr Polym 83:88–93
Falconer DJ, Mukerjea R, Robyt JF (2011) Biosynthesis of dextrans with different molecular weights by selecting the concentration of B-512FMC dextransucrase, the sucrose concentration, and the temperature. Carbohydr Res 346:280–284
Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ (2005) Leuconostoc dextransucrase and dextran: Production, properties and applications. J Chem Technol Biotechnol 80:845–860
Zhang Z, Liu Z, Tao X, Wei H (2016) Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr Polym 153:25–33
Purama RK, Goyal A (2008) Identification , effective purification and functional characterization of dextransucrase from Leuconostoc mesenteroides NRRL B-640. Bioresour Technol 99:3635–3642
Vettori MHPB, Blanco KC, Cortezi M et al (2012) Dextran: effect of process parameters on production, purification and molecular weight and recent applications. Diálogos & Ciência 2012:171–186
Qader SAU, Iqbal L, Aman A et al (2005) Production of dextran by newly isolated strains of Leuconostoc mesenteroides PCSIR-4 and PCSIR-9. Turkish J Biochem 31:21–26
Du R, Qiao X, Zhao F et al (2018) Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr Polym 198:529–536
Liu J, Wang X, Pu H et al (2017) Recent advances in endophytic exopolysaccharides: production, structural characterization, physiological role and biological activity. Carbohydr Polym 157:1113–1124
Du R, Xing H, Yang Y et al (2017) Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydr Polym 174:409–416
Brigham CJ, Kurosawa K, Rha C, Sinskey AJ (2011) Bacterial carbon storage to value added products. J Microb Biochem Technol:s3
Agrawal M, Shukla R, Goyal A (2011) UV mutagenesis of Leuconostoc mesenteroides NRRL B-640 for generation of a mutant (B-640 M) with hyper-producing dextransucrase activity. Curr Trends Biotechnol Pharm 5:1445–1453
Moussa TAA, Khalil NM (2012) Solid-state fermentation for the production of dextran from Saccharomyces cerevisiae and its cytotoxic effects. Life Sci J 9:2210–2218
Moosavi-nasab M, Gavahian M, Yousefi AR et al (2010) Fermentative production of dextran using food industry wastes. World Acad Sci Eng Technol 44:1043–1045
Behravan J, Fazly Bazzaz BS, Salimi Z (2003) Optimization of dextran production by Leuconostoc mesenteroides NRRL B-512 using cheap and local sources of carbohydrate and nitrogen. Biotechnol Appl Biochem 38:267
Esmaeilnejad-Moghadam B, Mokarram RR, Hejazi MA et al (2019) Low molecular weight dextran production by Leuconostoc mesenteroides strains: optimization of a new culture medium and the rheological assessments. Bioact Carbohydrates Diet Fibre 18:100181
Han J, Hang F, Guo B et al (2014) Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydr Polym 112:556–562
Onilude AA, Olaoye O, Fadahunsi IF et al (2013) Effects of cultural conditions on dextran production by Leuconostoc spp. Int Food Res J 20:1645–1651
Srichayet P, Limsangouan N, Puntapurt K (2000) The composition of sugarcane juice and production of granulated sugar. 2000
Wei P, Cheng C, Lin M et al (2017) Production of poly (malic acid) from sugarcane juice in fermentation by Aureobasidium pullulans: kinetics and process economics. Bioresour Technol 224:581–589
Kayalvizhi V, Antony U (2011) Microbial a nd physico-chemical changes in tomato juice subjected to pulsed electric field treatment. African J Agric Res 6:6348–6353
Singh R, Gaur R, K. Pandey P, et al (2018) A novel media optimized for production of pullulan in flask type fermentation system. Int J Curr Microbiol Appl Sci 7:53–61.
Tsuchiya H, Koepsell H, Corman J et al (1952) The effect of certain cultural factors on production of dextransucrase by Leuconostoc mesenteroides. North Reg Res Lab 64:521–526
Zarour K, Goretti M, Prieto A et al (2017) Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr Polym 174:646–657
Rao MA, Cooley HJ, Vitali AA (1984) Flow properties of concentrated juices at low temperatures. Food Technol
Farinazzo FS, Valente LJ, Almeida MB et al (2020) Characterization and antioxidant activity of an exopolysaccharide produced by Leuconostoc pseudomesenteroides JF17 from juçara fruits (Euterpe edulis Martius). Process Biochem 91:141–148
Choudhury AR, Sharma N, Prasad GS (2012) Deoiledjatropha seed cake is a useful nutrient for pullulan production. Microb Cell Fact 11:3–11
Lule VK, Singh R, Pophaly SD et al (2016) Production and structural characterisation of dextran from an indigenous strain of Leuconostoc mesenteroides BA08 in Whey. Int J Dairy Technol 69:520–531
Santos M, Teixeira J, Rodrigues A (2000) Production of dextransucrase, dextran and fructose from sucrose using Leuconostoc mesenteroides NRRL B512 ( f ). Biochem Eng J 4:177–188
Sarwat F, Qader SAU, Aman A, Ahmed N (2008) Production & characterization of a unique dextran from an indigenous Leuconostoc mesenteroides CMG713. Int J Biol Sci 4:379–386
Kanimozhi J, Moorthy IG, Sivashankar R, Sivasubramanian V (2017) Optimization of dextran production by Weissella cibaria NITCSK4 using response surface methodology-genetic algorithm based technology. Carbohydr Polym 174:103–110
Otts DR, Day DF (1988) Dextransucrase secretion in Leuconostoc mesenteroides depends on the presence of a transmembrane proton gradient. J Bacteriol 170:5006–5011
Lazić ML, Veljković VB, Vučetić JI, Vrvić MM (1993) Effect of pH and aeration on dextran production by Leuconostoc mesenteroides. Enzyme Microb Technol 15:334–338
Karthikeyan RS, Rakshit SK, Baradarajan A (1996) Optimization of batch fermentation conditions for dextran production. Bioprocess Eng 15:247–251
Vedyashkina TA, Revin VV, Gogotov IN (2005) Optimizing the conditions of dextran synthesis by the bacterium Leuconostoc mesenteroides grown in a molasses-containing medium. Appl Biochem Microbiol 41:409–413
Zhang ZY, Jin B, Kelly JM (2007) Production of lactic acid from renewable materials by Rhizopus fungi. Biochem Eng J 35:251–263
Srinivas B, Padma PN (2014) Screening Of Diverse Organic, Inorganic And Natural Nitrogen Sources For Dextran Production By Weissella Sps Using Plackett-Burman Design. 3:234–237
Abedin RM, El-Borai AM, Shall MA, El-Assar SA (2013) Optimization and statistical evaluation of medium components affecting dextran and dextransucrase production by Lactobacillus acidophilus ST76480.01. Life Sci J 10:1746–1753
Shukla S, Goyal A (2011) Optimization of fermentation medium for enhanced glucansucrase and glucan production from Weissella confusa. Brazilian Arch Biol Technol 54:1117–1124
Baruah R, Deka B, Kashyap N, Goyal A (2018) Dextran utilization during its synthesis by Weissella cibaria RBA12 can be overcome by fed-batch fermentation in a bioreactor. Appl Biochem Biotechnol 184
Majumder A, Bhandari S, Purama RK et al (2009) Enhanced production of a novel dextran from Leuconostoc mesenteroides NRRL B-640 by Response Surface Methodology. 59:309–315
Fattah AFA, Hashem AM, El-refai MA, Gebreel HM (2012) Production and properties of dextransucrase by free and immobilized cells of Leuconostoc paramesenteroides. Egypt Pharm J:42–48
Wang X, Xu P, Yuan Y et al (2006) Modeling for gellan gum production by. Microbiology 72:3367–3374
Hamieh A, Olama Z, Holail H (2013) Microbial production of polyhydroxybutyrate , a biodegradable plastic using agro-industrial waste. Glob Adv Res J Microbiol 2:54–64
Srinivas B, Padma PN (2015) Effect of process parameters on dextran production by Weissella Confusa. Int J Res Appl Sci Eng 3:373–377
Qader SAU, Aman A (2012) Low molecular weight dextran: Immobilization of cells of Leuconostoc mesenteroides KIBGE HA1 on calcium alginate beads. Carbohydr Polym 87:2589–2592
Devi N, Singh AK, Azmi W (2018) Analysis of the combined effects of aeration and agitation rate on dextransucrase production by Leuconostoc lactis KU665298 in a Laboratory Fermenter using Response Surface Methodology. J Microbiol Biotechnol 7:41–52
Baruah R, Deka B, Kashyap N (2017) Dextran utilization during its synthesis by Weissella cibaria RBA12 can be overcome by fed-batch fermentation in a bioreactor. Appl Biochem Biotechnol 184:1–11
Heilig ML (1994) United States Patent Office. ACM SIGGRAPH Comput Graph 28:131–134
Feng F, Zhou Q, Yang Y et al (2018) Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int J Biol Macromol 113:45–50
Robyt JF, Kimble BK, Walseth TF (1974) The mechanism of dextransucrase action. Direction of dextran biosynthesis. Arch Biochem Biophys 165:634–640
Youssef F, Roukas T, Biliaderis CG (1999) Pullulan production by a non-pigmented strain of Aureobasidium pullulans using batch and fed-batch culture. Process Biochem 34:355–366
Sugumaran KR, Ponnusami V (2017) Conventional optimization of aqueous extraction of pullulan in solid-state fermentation of Cassava bagasse and Asian palm kernel. Biocatal Agric Biotechnol 10:204–208
Huang J, Zhu S, Li C, et al (2019) Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep Biochem Biotechnol 0:1–7.
Prechtl RM, Wefers D, Jakob F, Vogel RF (2018) Cold and salt stress modulate amount, molecular and macromolecular structure of a Lactobacillus sakei dextran. Food Hydrocoll 82:73–81
Landon RS, Law RCS, Webb C (1993) Fermentation broth rheology during dextran production by Leuconostoc mesenteroides B512 ( F ) as a possible tool for control. Appl Microbiol Biotechnol 40:251–257
Tirtaatmadja V, Dunstan DE, Boger DV (2001) Rheology of dextran solutions. J Nonnewton Fluid Mech 97:295–301
Prasanna PHP, Bell A, Grandison AS, Charalampopoulos D (2012) Emulsifying, rheological and physicochemical properties of exopolysaccharide produced by Bifidobacterium longum subsp. infantis CCUG 52486 and Bifidobacterium infantis NCIMB 702205. Carbohydr Polym 90:533–540
Santos M, Rodrigues A, Teixeira JA (2005) Production of dextran and fructose from carob pod extract and cheese whey by Leuconostoc mesenteroides NRRL B512 ( f ). Biochem Eng J 25:1–6
Moosavi-nasab M, Alahdad Z, Nazemi S (2009) Characterization of the dextran produced by Leuconostoc mesenteroides from date fruit extract. Iran Agric Res 27:79–88
Mothé CG, Rao MA (1999) Rheological behavior of aqueous dispersions of cashew gum and gum arabic: effect of concentration and blending. Food Hydrocoll 13:501–506
Wang ZM, Cheung YC, Leung PH, Wu JY (2010) Ultrasonic treatment for improved solution properties of a high-molecular weight exopolysaccharide produced by a medicinal fungus. Bioresour Technol 101:5517–5522
Zhu KX, Huang S, Peng W et al (2010) Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res Int 43:943–948
Kavitake D, Devi PB, Singh SP, Shetty PH (2016) Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int J Biol Macromol 86:681–689
Zhou Q, Feng F, Yang Y et al (2017) Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int J Biol Macromol:1–8
Bertocchi C, Delneri D, Signore S et al (1997) Characterization of microbial cellulose from a high-producing mutagenized Acetobacter pasteurianus strain. Biochim Biophys Acta - Gen Subj 1336:211–217
Purama RK, Goswami P, Khan AT, Goyal A (2009) Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr Polym 76:30–35
Yang Y, Peng Q, Guo Y et al (2015) Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr Polym 133:365–372
Rosca I, Roxana A, Peptanariu D et al (2017) Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int J Biol Macromol.
Zamora F, González MC, Dueñas MT et al (2002) Thermodegradation and thermal transitions of an exopolysaccharide produced by Pediococcus damnosus 2.6. J Macromol Sci - Phys 41 B:473–486
Kenari HS, Imani M, Nodehi A (2013) Full factorial design-of-experiments for preparation of crosslinked dextran microspheres. J Appl Polym Sci 127:3712–3724
Miao M, Huang C, Jia X et al (2015) Physicochemical characteristics of a high molecular weight bioengineered α-D-glucan from Leuconostoc citreum SK24.002. Food Hydrocoll 50:37–43
Llamas-arriba MG, Puertas AI, Prieto A et al (2019) Characterization of dextrans produced by Lactobacillus mali CUPV271 and Leuconostoc carnosum CUPV411 María. Food Hydrocoll 89:613–622
Sun YX, Liu JC, Yang XD, Kennedy JF (2010) Purification, structural analysis and hydroxyl radical-scavenging capacity of a polysaccharide from the fruiting bodies of Russula virescens. Process Biochem 45:874–879
Acknowledgments
All authors are thankful to the Management SASTRA Deemed to be University, Thanjavur, Tamil Nadu, India, for providing the necessary facilities. Ms. Sameeha Syed Abdul Rahman, who is a research scholar, is a recipient of the DST – INSPIRE fellowship (IF180367) from the Department of Science and Technology, Government of India, and the support is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Rahman, S.S.A., Venkatachalam, P. & Karuppiah, S. Cost-effective production of dextran using Saccharum officinarum juice (SOJ) as a potential feedstock: downstream processing and characterization. Biomass Conv. Bioref. 12, 4863–4875 (2022). https://doi.org/10.1007/s13399-020-00926-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00926-4