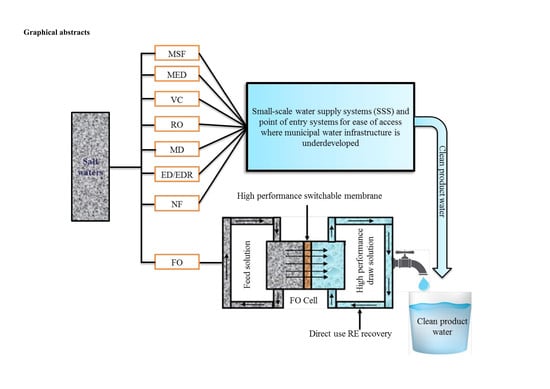
Seawater Desalination: A Review of Forward Osmosis Technique, Its Challenges, and Future Prospects
Abstract
:1. Introduction
2. Challenge of Global Water Scarcity
Global Freshwater Demands and Water Scarcity-Stress Situations
3. Seawater Desalination
3.1. Overview of Major Desalination Technologies
3.1.1. Thermal Seawater Desalination Technologies
3.1.2. Membrane Desalination Processes
3.2. Brief Insights—Energy and Economic Implications of Desalination
4. Forward Osmosis
4.1. Benefits and Applications of Forward Osmosis
4.2. Research Trends on FO Technology
4.3. FO Life Cycle
5. Forward Osmosis Desalination Process
5.1. Selection Criteria for Draw Solutes
5.2. Draw Solutes in Forward Osmosis Processes
5.2.1. Non-Responsive Draw Solutes
5.2.2. Responsive Draw Solutes and Synthetic Materials
5.3. FO Membrane Developments
5.3.1. Design Criteria of FO Membranes
5.3.2. FO Membrane Types
Cellulose Based Membranes
Thin-Film Composite Membranes
Carbon Based Membranes
5.3.3. Membrane Fouling
5.3.4. Concentration Polarization
5.3.5. Reverse Salt Flux
5.4. Draw Solutes Development and Draw Solutions Regeneration
5.5. Combination of FO with Other Technologies
Solar Energy Integrated FO Desalination
6. Challenges and Future Perspectives in FO Technology for Water Desalination
6.1. Design of Suitable DS
6.2. Engineered Fabrication of Membrane
6.3. Energy Efficiency
6.4. Cost Effective Desalination Systems
7. Conclusions
- the development of functional materials with the right mix of properties that maintains and sustains non-equilibrium in solute concentrations as draw solutes in FO processes could offer a solution to the challenges related to ideal DS;
- the challenge of membrane fouling in FO applications could potentially be addressed through fabrication of high permeability switchable membranes;
- research in direct solar FO desalination could potentially offer solution to achieving sustainable energy savings in FO desalination;
- small-scale system and point of entry systems could provide more opportunity to equitably guarantee future clean water supply in a situation of both lack and economic water scarcity.
Author Contributions
Funding
Conflicts of Interest
References
- Hameeteman, E. Future water. In Security: Facts, Figures and Predictions; Global Water Institute: Columbus, OH, USA, 2013. [Google Scholar]
- American-Membrane-Technology-Association. Water Desalination Processes. 2007. Available online: https://www.amtaorg.com/wp-content/uploads/08_Water_Desalination_Processes.pdf (accessed on 23 November 2019).
- Gao, L.; Yoshikawa, S.; Iseri, Y.; Fujimori, S.; Kanae, S. An economic assessment of the global potential for seawater desalination to 2050. Water 2017, 9, 763. [Google Scholar] [CrossRef]
- Connor, R. The world Water Development Report 2014: Water and Energy; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2014. [Google Scholar]
- Khan, M.; Almesfer, M.; Danish, M.; Ali, I.; Shoukry, H.; Patel, R.; Gardy, J.; Nizami, A.; Rehan, M. Potential of Saudi natural clay as an effective adsorbent in heavy metals removal from wastewater. Desalin. Water Treat. 2019, 158, 140–151. [Google Scholar] [CrossRef]
- Hesas, R.H.; Baei, M.S.; Rostami, H.; Gardy, J.; Hassanpour, A. An investigation on the capability of magnetically separable Fe3O4/mordenite zeolite for refinery oily wastewater purification. J. Environ. Manag. 2019, 241, 525–534. [Google Scholar] [CrossRef] [PubMed]
- United-Nations-World-Water-Assessment-Programme. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; Connor, R., Ed.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2018. [Google Scholar]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [Green Version]
- International-Desalination-Association. Desalination and Water Reuse by the Numbers. 2019. Available online: https://idadesal.org/ (accessed on 25 November 2019).
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- World-Bank. The Role of Desalination in an Increasingly Water-Scarce World; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Bazargan, A. A Multidisciplinary Introduction to Desalination; Stylus Publishing LLC: Sterling, VA, USA, 2018. [Google Scholar]
- AMTA. Membrane Desalination Power Usage Put in Perspective. 2016. Available online: https://www.amtaorg.com/wp-content/uploads/07_Membrane_Desalination_Power_Usage_Put_In_Perspective.pdf (accessed on 13 November 2019).
- Energy, U.R. Water Desalination Using Renewable Energy; IRENA: Abu Dhabi, UAE, 2012. [Google Scholar]
- Energy-Information-Administration, U.S. International Energy Outlook 2010; Energy Information Administration (eia): Washington, DC, USA, 2011; pp. 1–410.
- Li, Z.; Siddiqi, A.; Anadon, L.D.; Narayanamurti, V. Towards sustainability in water-energy nexus: Ocean energy for seawater desalination. Renew. Sustain. Energy Rev. 2018, 82, 3833–3847. [Google Scholar] [CrossRef] [Green Version]
- Hangzhou-Water. Forward Osmosis Desalination. 2017. Available online: https://www.modernwater.com/desalination-systems/forward-osmosis-desalination (accessed on 23 January 2020).
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward osmosis application in manufacturing industries: A short review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Tanganov, B.B. About sizes of the hydrated salt ions—The components of sea water. Eur. J. Nat. Hist. 2013, 1, 36–37. [Google Scholar]
- Zhu, H.; Zhang, L.; Wen, X.; Huang, X. Feasibility of applying forward osmosis to the simultaneous thickening, digestion, and direct dewatering of waste activated sludge. Bioresour. Technol. 2012, 113, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.S.; Zhang, S.; Wang, K.Y.; Su, J.; Ling, M.M. Forward osmosis processes: Yesterday, today and tomorrow. Desalination 2012, 287, 78–81. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [PubMed]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Forward osmosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Alihemati, Z.; Hashemifard, S.; Matsuura, T.; Ismail, A.; Hilal, N. Current status and challenges of fabricating thin film composite forward osmosis membrane: A comprehensive roadmap. Desalination 2020, 491, 114557. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Yangali-Quintanilla, V.; Ghaffour, N.; Amy, G.; Leiknes, T.; Vrouwenvelder, J.S. Life cycle cost of a hybrid forward osmosis—Low pressure reverse osmosis system for seawater desalination and wastewater recovery. Water Res. 2016, 88, 225–234. [Google Scholar]
- Nicoll, P. Forward Osmosis—A brief introduction. In Proceedings of the International Desalination Association World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. [Google Scholar]
- Modern-Water. Forward Osmosis: Desalination. 2013. Available online: https://www.modernwater.com/pdf/MW_Factsheet_Membrane_HIGHRES.pdf (accessed on 23 August 2019).
- Cuenca, J.C. Report on Water Desalination Status in the Mediterranean Countries; Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (IMIDA): La Alberca, Spain, 2013. [Google Scholar]
- Kim, T.W.; Kim, Y.; Yun, C.; Jang, H.; Kim, W.; Park, S.J.D. Systematic approach for draw solute selection and optimal system design for forward osmosis desalination. Desalination 2012, 284, 253–260. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T.J.D. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Borsani, R.; Rebagliati, S.J.D. Fundamentals and costing of MSF desalination plants and comparison with other technologies. Desalination 2005, 182, 29–37. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, H.; Shin, Y.; Jang, Y.; Lee, S.J.M. Economic evaluation of a hybrid desalination system combining forward and reverse osmosis. Membranes 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazlan, N.M.; Peshev, D.; Livingston, A.G.J.D. Energy consumption for desalination—A comparison of forward osmosis with reverse osmosis, and the potential for perfect membranes. Desalination 2016, 377, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Kang, Y.; Yang, C. Osmosis and its Applications; Encyclopedia of Microfluidics and Nanofluidics; Springer: Boston, MA, USA, 2013; pp. 1–14. [Google Scholar]
- Zhao, Q.; Chen, N.; Zhao, D.; Lu, X. Thermoresponsive magnetic nanoparticles for seawater desalination. ACS Appl. Mater. Interfaces 2013, 5, 11453–11461. [Google Scholar] [CrossRef] [PubMed]
- Al-aibi, S.; Mahood, H.B.; Sharif, A.O.; Alpay, E.; Simcoe-Read, H. Evaluation of draw solution effectiveness in a forward osmosis process. Desalin. Water Treat. 2016, 57, 13425–13432. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abdulrahim, H.K.; Hassan, A.S.; Mabrouk, A.A.; Sharif, A.O. The forward osmosis and desalination. Desalin. Water Treat. 2016, 57, 4269–4295. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S. A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: Evaluating the performance of fertilizer draw solutions. J. Membr. Sci. 2011, 375, 172–181. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Shon, H.K.; Vigneswaran, S.; Kandasamy, J.; Chanan, A. A review of draw solutes in forward osmosis process and their use in modern applications. Desalin. Water Treat. 2012, 43, 167–184. [Google Scholar] [CrossRef]
- Corzo, B.; de la Torre, T.; Sans, C.; Ferrero, E.; Malfeito, J.J. Evaluation of draw solutions and commercially available forward osmosis membrane modules for wastewater reclamation at pilot scale. Chem. Eng. J. 2017, 326, 1–8. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Q.; Zhang, T.C.; Tao, T.; Zhou, A.; Chen, L.; Bie, X. A review on the recovery methods of draw solutes in forward osmosis. J. Water Process Eng. 2014, 4, 212–223. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, M.; Zou, S.; Yang, X.; Long, T.E.; He, Z. Efficient recovery of polyelectrolyte draw solutes in forward osmosis towards sustainable water treatment. Desalination 2017, 422, 134–141. [Google Scholar] [CrossRef]
- Amjad, M.; Gardy, J.; Hassanpour, A.; Wen, D. Novel draw solution for forward osmosis based solar desalination. Appl. Energy 2018, 230, 220–231. [Google Scholar] [CrossRef]
- Phuntsho, S.; Sahebi, S.; Majeed, T.; Lotfi, F.; Kim, J.E.; Shon, H.K. Assessing the major factors affecting the performances of forward osmosis and its implications on the desalination process. Chem. Eng. J. 2013, 231, 484–496. [Google Scholar] [CrossRef]
- Alnaizy, R.; Aidan, A.; Qasim, M. Copper sulfate as draw solute in forward osmosis desalination. J. Environ. Chem. Eng. 2013, 1, 424–430. [Google Scholar] [CrossRef]
- Adham, S.; Oppenheimer, J.; Liu, L.; Kumar, M. Dewatering Reverse Osmosis Concentrate from Water Reuse Using forward Osmosis; WateREUES: Alexandria, VA, USA, 2007; pp. 1–52. [Google Scholar]
- Bai, H.; Liu, Z.; Sun, D.D. Highly water soluble and recovered dextran coated Fe3O4 magnetic nanoparticles for brackish water desalination. Sep. Purif. Technol. 2011, 81, 392–399. [Google Scholar] [CrossRef]
- Ge, Q.; Su, J.; Chung, T.S.; Amy, G. Hydrophilic superparamagnetic nanoparticles: Synthesis, characterization, and performance in forward osmosis processes. Ind. Eng. Chem. Res. 2011, 50, 382–388. [Google Scholar] [CrossRef]
- Ling, M.M.; Wang, K.Y.; Chung, T.S. Highly water-soluble magnetic nanoparticles as novel draw solutes in forward osmosis for water reuse. Ind. Eng. Chem. Res. 2010, 49, 5869–5876. [Google Scholar] [CrossRef]
- Ling, M.M.; Chung, T.S. Desalination process using super hydrophilic nanoparticles via forward osmosis integrated with ultrafiltration regeneration. Desalination 2011, 278, 194–202. [Google Scholar] [CrossRef]
- Ling, M.M.; Chung, T.S. Novel dual-stage FO system for sustainable protein enrichment using nanoparticles as intermediate draw solutes. J. Membr. Sci. 2011, 372, 201–209. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Yao, J.; Simon, G.P.; Wang, H. Stimuli-responsive polymer hydrogels as a new class of draw agent for forward osmosis desalination. Chem. Commun. 2011, 47, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.L.; Rae, C.; Stewart, F.F.; Wilson, A.D. Switchable polarity solvents as draw solutes for forward osmosis. Desalination 2013, 312, 124–129. [Google Scholar] [CrossRef]
- Li, L.; Shi, W.; Yu, S. Research on forward osmosis membrane technology still needs improvement in water recovery and wastewater treatment. Water 2020, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Porifera. Technology. A Powerful Pairing of Forward Osmosis and Unique Draw Regeneration Solutions for Optimal Concentration and Purification. pp. 179–181. Available online: http://www.porifera.com/technology (accessed on 18 March 2020).
- TIDAL™. TIDAL™ Forward Osmosis. 2020. Available online: https://www.kochmembrane.com/en-US/products/spiral-membranes/TIDAL-Forward-Osmosis (accessed on 18 March 2020).
- Im, S.J.; Choi, J.; Jeong, S.; Jang, A. New concept of pump-less forward osmosis (FO) and low-pressure membrane (LPM) process. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, P.S.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S. Recent progresses of forward osmosis membranes formulation and design for wastewater treatment. Water 2019, 11, 2043. [Google Scholar] [CrossRef] [Green Version]
- Hydroxsys. Revolutionising Global Filtration Technology. Available online: https://www.hydroxsys.com/ (accessed on 18 March 2020).
- Xu, W.; Chen, Q.; Ge, Q. Recent advances in forward osmosis (FO) membrane: Chemical modifications on membranes for FO processes. Desalination 2017, 419, 101–116. [Google Scholar] [CrossRef]
- Sreedhar, I.; Khaitan, S.; Gupta, R.; Reddy, B.M.; Venugopal, A. An odyssey of process and engineering trends in forward osmosis. Environ. Sci. Water Res. Technol. 2018, 4, 129–168. [Google Scholar] [CrossRef]
- Ong, R.C.; Chung, T.S.; Helmer, B.J.; de Wit, J.S. Novel cellulose esters for forward osmosis membranes. Ind. Eng. Chem. Res. 2012, 51, 16135–16145. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Mulcahy, D. Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 2012, 284, 175–181. [Google Scholar] [CrossRef]
- Wang, Y.N.; Goh, K.; Li, X.; Setiawan, L.; Wang, R. Membranes and processes for forward osmosis-based desalination: Recent advances and future prospects. Desalination 2018, 434, 81–99. [Google Scholar] [CrossRef]
- Yang, E.; Chae, K.J.; Kim, I.S. Biotechnology. Comparison of different semipermeable membranes for power generation and water flux in osmotic microbial fuel cells. J. Chem. Technol. 2016, 91, 2305–2312. [Google Scholar]
- Chan, E.; Young, A.; Lee, J.H.; Stafford, C. Swelling of Ultrathin Molecular Layer-by-Layer Polyamide Water Desalination Membranes. J. Polym. Sci., Part B: Polym. Phys. 2013, 51. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. A new commercial thin film composite membrane for forward osmosis. J. Desalination. 2014, 343, 187–193. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Wang, Y. Substrate matters: The influences of substrate layers on the performances of thin-film composite reverse osmosis membranes. Chin. J. Chem. Eng. 2017, 25, 1676–1684. [Google Scholar] [CrossRef]
- Blandin, G.; Vervoort, H.; Le-Clech, P.; Verliefde, A.R.D. Fouling and cleaning of high permeability forward osmosis membranes. J. Water Process Eng. 2016, 9, 161–169. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Nghiem, L.D.; Li, X.M.; Xie, M.; Zhao, B.; Zhang, M.; Song, J.; He, T. Treatment of shale gas drilling flowback fluids (SGDFs) by forward osmosis: Membrane fouling and mitigation. Desalination 2015, 366, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Duong, P.H.H.; Chung, T.S. Application of thin film composite membranes with forward osmosis technology for the separation of emulsified oil–water. J. Membr. Sci. 2014, 452, 117–126. [Google Scholar] [CrossRef]
- Boretti, A.; Al-Zubaidy, S.; Vaclavikova, M.; Al-Abri, M.; Castelletto, S.; Mikhalovsky, S. Outlook for graphene-based desalination membranes. NPJ Clean Water 2018, 1, 5. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- Brodie, B.C., XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. Ser. A 1859, 149, 249–259. [Google Scholar]
- Hummers, W.S.O.; Richard, E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ali, W. Advances in forward osmosis membranes: Altering the sub-layer structure via recent fabrication and chemical modification approaches. Desalination 2018, 436, 176–201. [Google Scholar]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Choi, H.G.; Shah, A.; Nam, S.E.; Park, Y.I.; Park, H. Thin-film composite membranes comprising ultrathin hydrophilic polydopamine interlayer with graphene oxide for forward osmosis. Desalination 2018, 449, 41–49. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wu, K.F.; Wang, Z.J.; Zhao, L.; Li, S.S. Fouling and cleaning of membrane—A literature review. J. Water Process Eng. 2000, 12, 241–251. [Google Scholar]
- Li, L.; Liu, X.P.; Li, H.Q. A review of forward osmosis membrane fouling: Types, research methods and future prospects. Environ. Technol. Rev. 2017, 6, 26–46. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Bakly, S.; Khanafer, D.; Altaee, A.; Padmanaban, V.C.; Samal, A.K.; Hawari, A.H. Organic Fouling in Forward Osmosis: A Comprehensive Review. Water 2020, 12, 1505. [Google Scholar] [CrossRef]
- Lotfi, F.; Samali, B.; Hagare, D. Cleaning efficiency of the fouled forward osmosis membranes under different experimental conditions. J. Environ. Chem. Eng. 2018, 6, 4555–4563. [Google Scholar] [CrossRef]
- Semiat, R.; Sapoznik, J.; Hasson, D. Energy aspects in osmotic processes. Desalin. Water Treat. 2010, 15, 228–235. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L. Relating solution physicochemical properties to internal concentration polarization in forward osmosis. J. Membr. Sci. 2011, 379, 459–467. [Google Scholar] [CrossRef]
- Hancock, N.T.; Cath, T.Y. Solute coupled diffusion in osmotically driven membrane processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Phuntsho, S.; Zhang, T.C.; Surampalli, R.Y. Forward Osmosis: Fundamentals and Applications. 2015. Available online: http://ascelibrary.org/doi/book/10.1061/9780784414071 (accessed on 18 March 2020).
- Alejo, T.; Arruebo, M.; Carcelen, V.; Monsalvo, V.M.; Sebastian, V. Advances in draw solutes for forward osmosis: Hybrid organic-inorganic nanoparticles and conventional solutes. Chem. Eng. J. 2017, 309, 738–752. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Lauber, L.; Roest, K.; Harmsen, D.J.H.; Post, J.W.; Rietveld, L.C.; van Lier, J.B.; Cornelissen, E.R. Zwitterions as alternative draw solutions in forward osmosis for application in wastewater reclamation. J. Membr. Sci. 2014, 460, 82–90. [Google Scholar] [CrossRef]
- Hau, N.T.; Chen, S.S.; Nguyen, N.C.; Huang, K.Z.; Ngo, H.H.; Guo, W. Exploration of EDTA sodium salt as novel draw solution in forward osmosis process for dewatering of high nutrient sludge. J. Membr. Sci. 2014, 455, 305–311. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Quantick, P.; Petropakis, H. A study of the direct osmotic concentration of tomato juice in tubular membrane—module configuration. I. The effect of certain basic process parameters on the process performance. J. Membr. Sci. 1998, 150, 99–110. [Google Scholar] [CrossRef]
- Ng, H.; Tang, W. Forward (Direct) Osmosis: A Novel and Prospective Process for Brine Control. Proc. Water Environ. Fed. 2006, 2006, 4345–4352. [Google Scholar] [CrossRef]
- Ng, H.Y.; Tang, W.; Wong, W.S. Performance of Forward (Direct) Osmosis Process: Membrane Structure and Transport Phenomenon. Environ. Sci. Technol. 2006, 40, 2408–2413. [Google Scholar] [CrossRef]
- Yaeli, J. Method and Apparatus for Processing Liquid Solutions of Suspensions Particularly Useful in the Desalination of Saline Water. U.S. Patent US5098575A, 24 March 1992. [Google Scholar]
- Kravath, R.E.D.; Joanna, A. Desalination of sea water by direct osmosis. Desalination 1975, 16, 151–155. [Google Scholar] [CrossRef]
- Su, J.; Chung, T.S.; Helmer, B.J.; de Wit, J.S. Enhanced double-skinned FO membranes with inner dense layer for wastewater treatment and macromolecule recycle using Sucrose as draw solute. J. Membr. Sci. 2012, 396, 92–100. [Google Scholar] [CrossRef]
- McCormick, P.; Pellegrino, J.; Mantovani, F.; Sarti, G. Water, salt, and ethanol diffusion through membranes for water recovery by forward (direct) osmosis processes. J. Membr. Sci. 2008, 325, 467–478. [Google Scholar] [CrossRef]
- Bowden, K.S.; Achilli, A.; Childress, A.E. Organic ionic salt draw solutions for osmotic membrane bioreactors. Bioresour. Technol. 2012, 122, 207–216. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. A novel ammonia—carbon dioxide forward (direct) osmosis desalination process. Desalination 2005, 174, 1–11. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. Desalination by ammonia–carbon dioxide forward osmosis: Influence of draw and feed solution concentrations on process performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- Bachelder, G.W. Process for the Demineralization of Water. U.S. Patent US3171799A, 6 March 1965. [Google Scholar]
- Wallace, M.; Cui, Z.; Hankins, N.P. A thermodynamic benchmark for assessing an emergency drinking water device based on forward osmosis. Desalination 2008, 227, 34–45. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R.; Elimelech, M. Performance evaluation of sucrose concentration using forward osmosis. J. Membr. Sci. 2009, 338, 61–66. [Google Scholar] [CrossRef]
- Zou, S.; Gu, Y.; Xiao, D.; Tang, C.Y. The role of physical and chemical parameters on forward osmosis membrane fouling during algae separation. J. Membr. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Yen, S.K.; Haja N, F.M.; Su, M.; Wang, K.Y.; Chung, T.S. Study of draw solutes using 2-methylimidazole-based compounds in forward osmosis. J. Membr. Sci. 2010, 364, 242–252. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, P.; Wan, C.; Chung, T.S. Polyelectrolyte-promoted forward osmosis–membrane distillation (fo–md) hybrid process for dye wastewater treatment. Environ. Sci. Technol. 2012, 46, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Wang, Y.; Wang, H.; Xu, T. Thermo-sensitive polyelectrolytes as draw solutions in forward osmosis process. Desalination 2013, 318, 48–55. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Simon, G.P.; Wang, H. Forward osmosis desalination using polymer hydrogels as a draw agent: Influence of draw agent, feed solution and membrane on process performance. Water Res. 2013, 47, 209–215. [Google Scholar] [PubMed] [Green Version]
- Noh, M.; Mok, Y.; Lee, S.; Kim, H.; Lee, S.H.; Jin, G.W.; Seo, J.H.; Koo, H.; Park, T.H.; Lee, Y. Novel lower critical solution temperature phase transition materials effectively control osmosis by mild temperature changes. Chem. Commun. 2012, 48, 3845–3847. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, G.; Hankins, N. Assessment of Draw-Solution Performance in Combination with Asymmetric Membranes for Forward Osmosis; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Stone, M.L.; Wilson, A.D.; Harrup, M.K.; Stewart, F.F. An initial study of hexavalent phosphazene salts as draw solutes in forward osmosis. Desalination 2013, 312, 130–136. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, L.; Wang, K.; Yao, J.; Li, D.; Simon, G.P.; Wang, R.; Wang, H. Significantly enhanced water flux in forward osmosis desalination with polymer-graphene composite hydrogels as a draw agent. RSC Adv. 2013, 3, 887–894. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Al-Qaraghuli, A.; Kazmerski, L.L. Comparisons of technical and economic performance of the main desalination processes with and without renewable energy coupling. In Proceedings of the World Renewable Energy Forum, Denver, CO, USA, 13–17 May 2012; Boulder, C.O., Ed.; American Solar Energy Society (ASES), National Renewable Energy Lab. (NREL): Golden, CO, USA, 2012. [Google Scholar]
- Mahmoud, A.; Fath, H.; Ahmed, M. Enhancing the performance of a solar driven hybrid solar still/humidification-dehumidification desalination system integrated with solar concentrator and photovoltaic panels. Desalination 2018, 430, 165–179. [Google Scholar] [CrossRef]
- Arunkumar, T.; Jayaprakash, R.; Denkenberger, D.; Ahsan, A.; Okundamiya, M.S.; Kumar, S.; Tanaka, H.; Aybar, H.Ş. An experimental study on a hemispherical solar still. Desalination 2012, 286, 342–348. [Google Scholar] [CrossRef]
- Chen, T.C.; Ho, C.D. Immediate assisted solar direct contact membrane distillation in saline water desalination. J. Membr. Sci. 2010, 358, 122–130. [Google Scholar] [CrossRef]
- Frantz, C.; Seifert, B. Thermal analysis of a multi effect distillation plant powered by a solar tower plant. Energy Procedia 2015, 69, 1928–1937. [Google Scholar] [CrossRef] [Green Version]
- Banat, F.; Jumah, R.; Garaibeh, M. Exploitation of solar energy collected by solar stills for desalination by membrane distillation. Renew. Energy 2002, 25, 293–305. [Google Scholar] [CrossRef]
- Darawsheh, I.; Islam, M.D.; Banat, F. Experimental characterization of a solar powered MSF desalination process performance. Therm. Sci. Eng. Prog. 2019, 10, 154–162. [Google Scholar] [CrossRef]
- Omara, Z.M.; Eltawil, M.A.; ElNashar, E.A. A new hybrid desalination system using wicks/solar still and evacuated solar water heater. Desalination 2013, 325, 56–64. [Google Scholar] [CrossRef]
- Salcedo, R.; Antipova, E.; Boer, D.; Jiménez, L.; Guillén-Gosálbez, G. Multi-objective optimization of solar Rankine cycles coupled with reverse osmosis desalination considering economic and life cycle environmental concerns. Desalination 2012, 286, 358–371. [Google Scholar] [CrossRef]
- Kershman, S.A.; Rheinländer, J.; Gabler, H. Seawater reverse osmosis powered from renewable energy sources—Hybrid wind/photovoltaic/grid power supply for small-scale desalination in Libya. Desalination 2003, 153, 17–23. [Google Scholar] [CrossRef]
- Shaobo, H.; Zhang, Z.; Huang, Z.; Xie, A. Performance optimization of solar multi-stage flash desalination process using Pinch technology. Desalination 2008, 220, 524–530. [Google Scholar] [CrossRef]
- Schrier, J. Ethanol concentration by forward osmosis with solar-regenerated draw solution. Sol. Energy 2012, 86, 1351–1358. [Google Scholar] [CrossRef]
- Ghaffour, N.; Lattemann, S.; Missimer, T.; Ng, K.C.; Sinha, S.; Amy, G. Renewable energy-driven innovative energy-efficient desalination technologies. Appl. Energy 2014, 136, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Khayet, M.; Sanmartino, J.A.; Essalhi, M.; García-Payo, M.C.; Hilal, N. Modeling and optimization of a solar forward osmosis pilot plant by response surface methodology. Sol. Energy 2016, 137, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Khaydarov, R.A.; Khaydarov, R.R. Solar powered direct osmosis desalination. Desalination 2007, 217, 225–232. [Google Scholar] [CrossRef]
- Shrivastava, A.; Rosenberg, S.; Peery, M. Energy efficiency breakdown of reverse osmosis and its implications on future innovation roadmap for desalination. Desalination 2015, 368, 181–192. [Google Scholar] [CrossRef]
- Altaee, A.; Alanezi, A.A.; Hawari, A.H. 2—Forward osmosis feasibility and potential future application for desalination. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Waltham, MA, USA, 2018; pp. 35–54. [Google Scholar]
- Zhao, N.; Wang, H.; He, Z.; Yan, Q. Ammonia removal and recovery from diluted forward osmosis draw solution by using a tubular microbial desalination cell. Environ. Sci. Water Res. Technol. 2019, 5, 224–230. [Google Scholar] [CrossRef]












| Desalination Method * | Capital Costs (Million US$/MLD) | O&M (US$/m3) | Cost of Water Production (US$m3) | ||||
|---|---|---|---|---|---|---|---|
| Range | Average | Range | Average | Range | Average | ||
| MSF | 1.7–3.1 | 2.1 | 0.22–0.30 | 0.26 | 1.02–1.74 | 1.44 | |
| MED–TVC | 1.2–2.3 | 1.4 | 0.11–0.25 | 0.14 | 1.12–1.50 | 1.39 | |
| SWRO Mediterranean Sea | 0.8–2.2 | 1.2 | 0.25–0.74 | 0.35 | 0.64–1.62 | 0.98 | |
| SWRO Arabian Gulf | 1.2–1.8 | 1.5 | 0.36–1.01 | 0.64 | 0.96–1.92 | 1.35 | |
| SWRO Red Sea | 1.2–2.3 | 1.5 | 0.41–0.96 | 0.51 | 1.14–1.70 | 1.38 | |
| SWRO Atlantic and Pacific oceanic | 1.3–7.6 | 4.1 | 0.71–0.41 | 0.21 | 0.88–2.86 | 1.82 | |
| Hybrid | MSF/MED | 1.5–2.2 | 1.8 | 0.41–0.25 | 0.23 | 0.95–1.37 | 1.15 |
| SWRO | 1.2–2.4 | 1.3 | 0.29–0.44 | 0.35 | 0.85–1.12 | 1.03 | |
| Rank | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| 1 MIGD *, Steam price: 0.008$/MJ | Draw solute | C4H8O | NH4OH | C3H6O2 | NH4HCO3 | C2H6O | CH4O | C3H8O |
| Total cost ($/t) | 1.372 | 2.010 | 3.837 | 3.959 | 4.201 | 4.512 | 4.609 | |
| 100 MIGD, Steam price: 0.008$/MJ | Draw solute | C4H8O | NH4OH | C3H6O2 | NH4HCO3 | C2H6O | CH4O | C3H8O |
| Total cost ($/t) | 0.955 | 1.620 | 3.264 | 3.459 | 3.854 | 4.060 | 4.254 | |
| 1 MIGD, Steam price: 0.0001$/MJ | Draw solute | NH4OH | CH4O | C2H6O | C4H8O | NH4HCO3 | C3H8O | C3H6O2 |
| Total cost ($/t) | 0.275 | 0.343 | 0.344 | 0.348 | 0.395 | 0.402 | 0.545 | |
| 100 MIGD, Steam price: 0.0001$/MJ | Draw solute | NH4OH | NH4HCO3 | C4H8O | CH4O | C2H6O | C3H8O | C3H6O2 |
| Total cost ($/t) | 0.092 | 0.093 | 0.112 | 0.120 | 0.121 | 0.137 | 0.388 | |
| Company | Material and Type | Configuration | Status | Reference |
|---|---|---|---|---|
| Aquaporin A/S (AQP) | Biometric aquaporin | Flat sheet, Hollow fibre | Commercial | [59] |
| Fluid Technology Solutions | Cellulose triacetate | Flat sheet | Development | |
| Oasys Water | Thin film composite (TFC) | Flat sheet | Engineering application | |
| Nitto Denko | Composite membrane | Flat sheet, Hollow fibre | Commercial | |
| Woongjin Chemical Company Ltd. | Composite membrane | Flat sheet, Hollow fibre | Commercial | |
| Samsung Co. Ltd. | Composite membrane | Flat sheet | Development | |
| Porifera | TFC | Flat sheet | Commercial | [60] |
| Koch | Proprietary | Spiral wound | Commercial | [61] |
| Toyobo | Proprietary | Hollow fibre | Commercial | [29] |
| Toray | TFC | Spiral wound | Development | [29,62,63] |
| HydroxsysTM | Polyethylene | Not reported | Pre-commercial | [64] |
| Draw Solution Category | Draw Solution | Osmotic Pressure | Water Flux (Feed Solution & Membrane Type Employed) | Recovery Process(es) Employed | Drawbacks | References |
|---|---|---|---|---|---|---|
| Organic compounds | Glycine betaine (1.40 mol/kg) Glycine (1.24 mol/kg) L-proline (1.27 mol/kg) | 2.35 MPa 2.42 MPa 2.64 MPa | 4.83±0.15 L/m2h 4.59±0.38 L/m2h 4.31±0.57 L/m2h Deionised (DI) water/CTA | Anaerobic digestion | Severe dilutive ICP, Potentially cost intensive DS replacement, Biodegradation of DS and loss of DS via reverse salt flux. High membrane bio-fouling potential | [95] |
| EDTA sodium | 0.55 MPa (0.1 to 1.0 M) | 4.02 to 13.08 L/m2.h (Activated sludge—CTA cartridge type membrane) | Nanofiltration | Potential membrane impairment due to sludge deposition | [96] | |
| Glucose (C6H12O6) | 5.6 MPa (2.0 M) | 0.37 L/m2h (tomato juice—TFC aromatic polyamide) | Direct application | ICP effects necessitated by high molecular sizes of draw solutes | [97,98,99,100,101] | |
| Fructose (C6H12O6) | 5.6 MPa (2.0 M) | 2.5 L/m2h (0.5 M NaCl—thin layer cotton-derived cellulose-ester plastics embedded on to of a microfiltration membrane | Direct application | [98,99] | ||
| Sucrose (C12H12O11) | 2.7 MPa (1.0 M) | 12.9 L/m2h (DI water—CA hollow fiber) | Nanofiltration | Low water flux | [102] | |
| Ethanol (C2H6O) | 5.1 MPa (2.0 M) | Unstated | Pervaporation-based separation | High reverse salt flux and low water flux | [103] | |
| Sodium formate (HCOONa) | 31.4 MPa (at saturation) | 2.6 µm/s at 2.8 MPa (DI water—CTA flat sheet) | RO process | High reverse salt flux, Potentially cost intensive DS replacement | [104] | |
| Sodium acetate (C2H3NaO2) | 27.0 MPa (at saturation) | 2.5 µm/s at 2.8MPa (DI water—CTA flat sheet) | Potentially cost intensive DS replacement relative to inorganic salts | |||
| Sodium propionate (C3H5NaO2) | N/A | 2.41 µm/s at 2.8 MPa (DI water—CTA flat sheet) | ||||
| Magnesium acetate (Mg(CH3COO)2) | 10.3 MPa (at saturation) | 2.25 µm/s at 2.8 MPa (DI water—CTA flat sheet) | ||||
| HN(Me)2Cy HCO3 | ~33 MPa (7.6 mol/kg) | 10 L/m2h at 7.6 mol/kg (2 mol/kg NaCl—CTA flat sheet) | CO2 induced phase separation | Degradation of FO membrane | [58] | |
| Volatile compounds | Ammonium bicarbonate (NH4HCO3) | 6.7 MPa (2.0 M) | 2.04 µm/s (DI water—CTA flat sheet) | Heating—decomposition into NH3 and CO2 | Low solubility in water, High reverse salt flux, Potentially cost intensive DS replacement, Not thermally stable | [44,92,98,105,106] |
| Sulfur dioxide (SO2) | Not stated | Not stated | Heating air stripping or distillation | Volatile, Corrosive, Unstable in solution | [107] | |
| Nonresponsive draw solutes | Potassium chloride (KCl) | 9.1 MPa (2.0 M) | 6.337 µm/s (DI water—CA embedded in polyester woven mesh) | Direct application | High reverse salt flux | [41,44] |
| Sodium chloride (NaCl) | 10.2 MPa (2.0 M) | 2.68 µm/s (DI water—CTA flat sheet) | RO process, Distillation/RO, Direct application | High reverse salt flux | [44,97,108,109,110,111] | |
| Ammonium chloride (NH4Cl) | 8.9 MPa (2.0 M) | 5.348 µm/s (DI water—CA embedded in polyester woven mesh) | Direct application | High reverse salt flux | [41,44] | |
| Ammonium nitrate (NH4NO3) | 6.6 MPa (2.0 M) | 4.177 µm/s (DI water—CA embedded in polyester woven mesh) | Direct application | High reverse salt diffusion | [41] | |
| Potassium bromide (KBr) | 9.1 MPa (2.0 M) | 2.84 µm/s (DI water—CTA flat sheet) | RO process | Very high reverse salt diffusion, Potentially cost intensive DS replacement | [44] | |
| Sodium bicarbonate (NaHCO3) | 4.7 MPa (2.0) | 2.47 µm/s (DI water—CTA flat sheet | Low water solubility, Contain scale precursor ions | |||
| Potassium bicarbonate (KHCO3) | 8.0 MPa—(2.0 M) | 2.25 µm/s (DI water—CTA flat sheet) | Reverse salt flux, Contain scale precursor ions, Not easily recovered by RO | |||
| Magnesium chloride (MgCl2) | 26.0 MPa (2.0 M) | 2.33 µm/s (DI water—CTA flat sheet) | NF/direct application | Reverse salt flux, High viscosity Low diffusion coefficient, Mg2+ potential to effect membrane fouling via complexing with some functional groups | [44,108] | |
| Calcium chloride (CaCl2) | 22.1 MPa (2.0 M) | 2.64 µm/s (DI water—CTA flat sheet) | RO process | Reverse salt flux, Contain scale precursor ions, | [44,97,108] | |
| Ammonium sulphate ((NH4)2SO4) | 9.3 MPa (2.0 M) | 5.391 µm/s (DI water—CT embedded in polyester woven mesh) | Direct application | Reverse salt flux, Potentially cost intensive DS replacement | [41,44] | |
| Sodium sulphate (Na2SO4) | 9.7 MPa (2.0 M) | 2.14 µm/s (DI water—CTA flat sheet) | Nanofiltration | Reverse salt flux, Contain scale precursor ions | [44] | |
| Potassium sulphate (K2SO4) | 3.3 MPa (2.0 M) | 2.52 µm/s (DI water—CTA flat sheet) | RO process | Reverse salt flux, Low water solubility, Potentially cost intensive DS replacement. | ||
| Magnesium sulphate (MgSO4) | 5.6 MPa (2.0 M) | 1.54 µm/s (DI water—CTA flat sheet) | Nanofiltration | Reverse salt flux, High viscosity, Low water solubility, Contain scale precursor ions | ||
| Copper sulphate (CuSO4) | 3.0 MPa (220, 000 ppm) | 3.57 L/m2h (5, 050 ppm NaCl—CTA flat sheet) | Metathesis precipitation with barium hydroxide, and then sulphuric acid | Low water flux, FO process severely affected by concentration polarization effect | [50] | |
| Sodium nitrate (NaNO3) | 8.2 MPa (2.0 M) | 5.706 µm/s (DI water—CA embedded in polyester woven mesh) | Direct application | High reverse salt flux | [41] | |
| Potassium nitrate (KNO3) | 6.6 MPa (2.0 M) | 4.429 µm/s DI water CA embedded in polyester woven mesh) | High reverse salt flux Toxic, Energy intensive | |||
| Diammonium phosphate ((NH4)2HPO4) | 9.6 MPa (2.0 M) | 3.892 µm/s (DI water—CA embedded in polyester mesh) | Reverse slat flux, Low water flux | |||
| Ammonium phosphate (NH4H2PO4) | 7.7 MPa (2.0 M) | 4.349 µm/s (DI water—CA embedded in polyester mesh) | Reverse salt flux Low water flux | |||
| Calcium nitrate (Ca(NO3)2) | 11.0 MPa (2.0 M) | 50.22 µm/s DI water—CA embedded in polyester woven mesh) | Direct application | Potentially cost intensive DS replacement, Poor water extraction capacity | [41,44] | |
| Responsive draw solutes | Polyacrylic acid MNPs (PAA MNPs) | Up to 7.1 MPa (0.08mol/L) | 10 to 17 L/m2h (DI water—HTI membrane) | Magnetic field separation, Ultrafiltration | Drop in water flux due to agglomeration of MNPs | [54,55,56] |
| 2-pyrolidone based MNPs (2-pyrol MNPs) | Unstated | 0.5 to 5 L/m2h (DI water—HTI membrane) | ||||
| Triethylene glycol MNPs (TREG MNPs) | 0.5 to 5 L/m2h (DI water—HTI membrane) | |||||
| Polyethylene glycol diacid MNPs (PEG-(COOH)2MNPs) | 5.6 to 7.4 MPa (0.065 mol/L) | 5.3 to 9.1 L/m2h DI water—CTA flat sheet) | [53] | |||
| Nano size dextran coated ferric oxide MNPs (Fe3O4) | Unstated | 3.25 to 4 L/m2h (DI water—HTI membrane) | External magnet | [52] | ||
| 2-methylimidaxole based compounds with monovalent and divalent charges | 5.0 to 15 MPa (2.0 M) | 0.1 to 20 L/m2h (DI water—CTA flat sheet) | FO-MD integrated process | High ICP effect when using compound with divalent charge, High reverse solute flux, Potentially cost intensive DS replacement | [112] | |
| Polyelectrolyte of polyacrylic acid sodium (PAA-Na) | 2.5-4.6 MPa (0.72 mg/L) | 13 to 21 L/m2h (DI water—CA hollow fibre) | FO-MD integrated process, Ultrafiltration | Reverse salt flux, High viscosity | [113] | |
| Thermo-sensitive polyelectrolytes | Up to 8.9 MPa (14.28 wt.% polyelectrolytes with different sodium acrylate content) | 0.05 to 075 L/m2h (DI water—HTI membrane) | Hot ultrafiltration | Poor water flux | [114] | |
| Polymer hydrogels | 2.7 MPa | 0.55 to 1.1 L/m2h (2000 ppm NaCl—HTI membrane) | Direct application, Heating, Pressure stimuli | Energy intensive, Poor water flux | [57,115] | |
| Acyl-TAEA | N/A | N/A | High temperature | Poor water flux | [116] | |
| Micelles close to Kraft point | 9.5 MPa | 4.73 to 16.14 L/m2h (n.a) | Temperature swing with low grade heat and crystallization | Low diffusivity | [117] | |
| Dendrimers | 2279813 Pa (20 wt. %) | Unexamined | Wide range of pH value, and ultrafiltration | Somewhat inexpedient in practice | [51] | |
| Albumin | 4.8 MPa (30 wt. %) | Denatured and solidified upon heating | ||||
| Concentrated RO brines | Unmeasured | 8.8 to 11 L/m2h | RO process | Precipitation of organic salts on membrane surface | [55] | |
| Hexavalent phosphate | Unmeasured | 6 (Na salt) to 7 (Li salt) L/m2h (DI water—HTI membrane) | Direct application | Hydrolysis CTA membrane | [118] | |
| Carbon based nanoparticles | Polymer-graphene composite hydrogels | Unmeasured | 6.8 to 8.2 L/m2h (DI water/200 ppm NaCl—HTI membrane) | Heating | Poor water flux | [119] |
| Potassium carbon nanofibers (TEG-K/CNF) | 2.8 to 7.0 MPa (0.05 to 0.2 wt. %) | 10.5 to 13.3 L/m2h (3.0 wt.% NaCl—CTA flat sheet) | Solar evaporation | Decline in water flux | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aende, A.; Gardy, J.; Hassanpour, A. Seawater Desalination: A Review of Forward Osmosis Technique, Its Challenges, and Future Prospects. Processes 2020, 8, 901. https://doi.org/10.3390/pr8080901
Aende A, Gardy J, Hassanpour A. Seawater Desalination: A Review of Forward Osmosis Technique, Its Challenges, and Future Prospects. Processes. 2020; 8(8):901. https://doi.org/10.3390/pr8080901
Chicago/Turabian StyleAende, Aondohemba, Jabbar Gardy, and Ali Hassanpour. 2020. "Seawater Desalination: A Review of Forward Osmosis Technique, Its Challenges, and Future Prospects" Processes 8, no. 8: 901. https://doi.org/10.3390/pr8080901