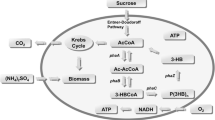
Abstract
This work discusses the optimization of the biopolymer PHAs production by Ralstonia eutropha, in a bioreactor carried out under fed-batch mode. Although the optimization of fed-batch fermentations involves the manipulation of the substrate feed rate, which generates a singular optimal control problem, the optimal trajectory can be also set by adjusting small segments by non-linear programming. The cybernetic structured mathematical model used here in is described by a system of 12 differential equations; the strategy involves the maximization/minimization of an Objective Function considering the model as a set of implicit constraints and the discretization of the manipulated variables (substrate feed rates). The sequential quadratic program method is used to solve the optimization problem. PHAs productivity is taken as the objective function and its results are compared to those documented in the literature.




Similar content being viewed by others
Abbreviations
- α1 :
-
Specific enzyme 1 synthesis rate (1/h)
- \(\alpha_{1}^{*}\) :
-
Specific enzyme 1 constitutive synthesis rate (1/h)
- α2 :
-
Specific enzyme 2 synthesis rate (1/h)
- \(\alpha_{2}^{*}\) :
-
Specific enzyme 2 constitutive synthesis rate (1/h)
- β1 :
-
Specific decay rate of enzyme 1 (1/h)
- β2 :
-
Specific decay rate of enzyme 2 (1/h)
- μ1,max :
-
Maximum specific rate of active biomass formation (1/h)
- μ2,max :
-
Maximum specific rate of PHB product formation (1/h)
- μ3,max :
-
Maximum specific rate of PHV product formation (1/h)
- E1 :
-
Enzyme 1 concentration (g/L)
- E2 :
-
Enzyme 2 concentration (g/L)
- F:
-
Global flow rate (g/h) (*)
- F1 :
-
Feed flow rate (g/h)
- F2 :
-
PH control flow rate (g/h) (**)
- KmS1 :
-
Limitation constant of glucose in cellular maintenance (g/L)
- KmS2 :
-
Limitation constant of fructose in cellular maintenance (g/L)
- KmS3 :
-
Limitation constant of nitrogen in cellular maintenance (g/L)
- KmS4 :
-
Limitation constant of oxygen in cellular maintenance (mg/L)
- KmS5 :
-
Limitation constant of propionic acid in cellular maintenance (g/L)
- \({\text{k}}_{{\text{L}}}{{\text{a}}}\) :
-
Oxygen mass transfer coefficient (1/h)
- KP :
-
Inhibition constant 1 of product formation by the content of intracellular polymer
- K0 :
-
Lag phase constant 1 for the beginning of the residual biomass formation phase
- K1 :
-
Lag phase constant 2 for the beginning of the residual biomass formation phase (h)
- K1C :
-
Limitation constant of active biomass formation rate by carbon (g/L)
- \({\text{K}}_{{1{\text{C}}}}^{{\text{E}}}\) :
-
Limitation constant of specific enzyme 1 synthesis rate by carbon (g/L)
- K1Ci :
-
Inhibition constant of active biomass formation rate by carbon (g/L)
- K13 :
-
Limitation constant of active biomass formation rate by nitrogen (g/L)
- \({\text{K}}_{13}^{{\text{E}}}\) :
-
Limitation constant of specific enzyme 1 synthesis rate by nitrogen (g/L)
- K14 :
-
Limitation constant of active biomass formation rate by oxygen (mg/L)
- \({\text{K}}_{14}^{{\text{E}}}\) :
-
Limitation constant of specific enzyme 1 synthesis rate by oxygen (mg/L)
- K2C :
-
Limitation constant of PHB product formation rate by carbon (g/L)
- \({\text{K}}_{{2{\text{C}}}}^{{\text{E}}}\) :
-
Limitation constant of specific enzyme 2 synthesis rate by carbon (g/L)
- K2Ci :
-
Inhibition constant of PHB product formation rate by carbon (g/L)
- K24 :
-
Limitation constant of PHB product formation rate by oxygen (mg/L)
- \({\text{K}}_{24}^{{\text{E}}}\) :
-
Limitation constant of specific enzyme 2 synthesis rate by oxygen (mg/L)
- K24i :
-
Inhibition constant of PHB product formation rate by oxygen (mg/L)
- K25i :
-
Inhibition constant of PHB product formation rate by propionic acid (g/L)
- K34 :
-
Limitation constant of PHV product formation rate by oxygen (mg/L)
- K34i :
-
Inhibition constant of PHV product formation rate by oxygen (mg/L)
- K35 :
-
Limitation constant of PHV product formation rate by propionic acid (g/L)
- K35i :
-
Inhibition constant of PHV product formation rate by propionic acid (g/L)
- mS1 :
-
Glucose consumption coefficient due to maintenance (1/h)
- mS2 :
-
Fructose consumption coefficient due to maintenance (1/h)
- mS3 :
-
Nitrogen consumption coefficient due to maintenance (1/h)
- mS4 :
-
Oxygen consumption coefficient due to maintenance (1/h)
- mS5 :
-
Propionic acid consumption coefficient due to maintenance (1/h)
- n24 :
-
Constant of PHB production inhibition by oxygen
- n34 :
-
Constant of PHV production inhibition by oxygen
- nP :
-
Constant of inhibition of products formation by the content of intracellular polymer
- n25i :
-
Inhibition constant of PHB product formation rate by propionic acid
- P1 :
-
PHB polymer (g/L)
- P2 :
-
PHV polymer (g/L)
- S1 :
-
Glucose concentration in the reactor (g/L)
- S2 :
-
Fructose concentration in the reactor (g/L)
- S3 :
-
Nitrogen concentration in the reactor (g/L)
- S4 :
-
Dissolved oxygen concentration (mg/L)
- \({\text{S}}_{4}^{*}\) :
-
Concentration of dissolved oxygen at 100% saturation (mg/L)
- S5 :
-
Propionic acid concentration in the reactor (g/L)
- S1e :
-
S1 feed concentration (g/L)
- S2e :
-
S2 feed concentration (g/L)
- S3e :
-
S3 feed concentration (g/L)
- S5e :
-
S5 feed concentration (g/L)
- \({\text{u}}_{1}^{*}\) :
-
Maximum value of the cybernetic variable that controls E1 synthesis
- V:
-
Volume (L)
- X:
-
Total biomass (g/L)
- Xr :
-
Active biomass (g/L)
- YP1S1 :
-
Glucose to PHB yield (g/g)
- YP1S2 :
-
Fructose to PHB yield (g/g)
- YP1S4 :
-
Oxygen to PHB yield (g/mg)
- YP1S5 :
-
Propionic acid to PHB yield (g/g)
- YP2S1 :
-
Glucose to PHV yield (g/g)
- YP2S2 :
-
Fructose to PHV yield (g/g)
- YP2S4 :
-
Oxygen to PHV yield (g/mg)
- YP2S5 :
-
Propionic acid to PHV yield (g/g)
- YXrS1 :
-
Glucose to active biomass yield (g/g)
- YXrS2 :
-
Fructose to active biomass yield (g/g)
- YXrS3 :
-
Nitrogen to active biomass yield (g/g)
- YXrS4 :
-
Oxygen to active biomass yield (g/mg)
- YXrS5 :
-
Propionic acid to active biomass yield (g/g)
References
Augusto EFP, Bonomi A, Giudici R (1994) Estratégias para ajuste de parâmetros em modelos de processos fermentativos inibidos pelo substrato e produto. In: 10. Congresso Brasileiro de Engenharia Química COBEQ, 1994. Anais. São Paulo SP Brasil. vol. 2. p. 1252–1257
Bradstreet RB (1965) The Kjeldahl method for organic nitrogen. Academic Press, London
Campos CD (2016) Plásticos—Tendências e Perspectivas (4 Jul 2016). Disponível em: https://reviplast.wordpress.com/2016/07/04/plsticos-tendncias-e-perspetivas/. Acesso em: 8 Nov 2017
European Bioplastics (2018) Disponível em: https://www.european-bioplastics.org/new-market-data-the-positive-trend-for-the-bioplastics-industry-remains-stable/
Ferraz L (1999) Proposição de um Modelo Fenomenológico Estruturado Cibernético para o Processo de Produção de Copolímero de Polihidroxialcanoatos. Master Thesis in Chemical Engineering. Escola Politécnica da USP
Gomez JGC, Rodrigues MFA, Alli RCP, Torres BB, Bueno Netto CL, Oliveira MS, da Silva LF (1996) Acid evaluation of soil gram-negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic. Appl Microbiol Biotechnol 45:785–791
Himmelblau E (1972) Apllied nonlinear programming. McGraw-Hill, Inc., New York
Kahaner D (1989) Numerical methods and Software. Pretice Hall, New Jersey
Kraft D (1994) Algorithm 733: TOMP—Fortran modules for optimal control calculations. ACM Trans Mathemat Softw 20(3):262–281
Lee YW, Yoo YJ (1994) High cell density culture of Alcaligenes eutrophus and PHB production by optimization of medium compositions. Korean J Appl Microbiol Biotechnol Lett 14:811–816
Lee Y, Kim M, Kim K, Kim GJ, Chang HN, Park YH (1995) Production of poly(β-hidroxybutyrate-co-β-hidroxivalerate) from glucose and valerate in Alcaligenes eutrophus. Biotech Lett 17(6):571–574
Lee LH et al (1997) Experimental optimization of fed-batch culture for poly-β-hydroxybutyric acid production. Biotechnol Bioeng 56(6):697–705
Lin YY (2017) Chen PT (2017) Development of polyhydroxybutyrate biosynthesis in Bacillus subtilis with combination of PHB-associated genes derived from Ralstonia eutropha and Bacillus megaterium. J Taiwan Inst Chem Eng 79:110–115
Microsoft PWB compiler version 1.10 (1990)
Piccoli R (1999) Otimização da Produção de Copolímeros de Polihidroxialcanoatos por Via Fermentativa, Baseada num Modelo Matemático Estruturado. Documento para Exame de Qualificação. Programa de Doutorado em Engenharia Química, Escola Politécnica da USP
Raicher G (2011) Análise econômica da produção de polímeros biodegradáveis no contexto de uma biorefinaria a partir de cana-de-açucar. Doctoral dissertation, USP-IPT-IBUTANTAN
Riis V, Mai W (1988) Gas chromatographic determination of poly-p-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J Chromatogr 445:285–289
Seoane IT et al (2015) Properties and processing relationship of polyhydroxybutyrate and cellulose biocomposites. Procedia Mat Sci 8:807–813
Sonnleitner B, Locher G, Fiechter A (1992) Biomass Determintation. J Biotechnol 25:5–22
Acknowledgments
Rosane A. M. Piccoli thanks the State of São Paulo Research Foundation for the doctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piccoli, R.M., Quiroz, L.H.C., de Toledo Fleury, A. et al. Optimization of polyhydroxyalkanoates bioproduction, based on a cybernetic mathematical model. Braz. J. Chem. Eng. 37, 643–652 (2020). https://doi.org/10.1007/s43153-020-00047-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00047-5