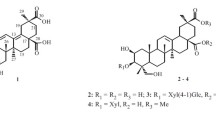
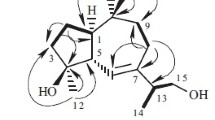
A new sesquiterpene lactone, namely, eupachiilide A (1), as well as two known sesquiterpene lactones, eupachinilide B (2), and eupalinilide G (3), were isolated from the whole plant of Eupatorium chinense L. The new structure was elucidated by spectral methods, especially 2D NMR techniques. The three natural compounds were all Michael addition acceptors, whose electrophilic moiety could react with the nucleophilic residues of the relevant active site and produce a variety of bioactivities. Eupachiilide A demonstrated potent cytotoxicity against two TNBC cell lines.

Similar content being viewed by others
References
S. Narrandes, S. Huang, L. Murphy, and W. Xu, BMC Cancer, 18, 22 (2018).
S. Hurvitz and M. Mead, Curr. Opin. Obstet. Gynecol., 28, 59 (2016).
I. V. Maucher, M. Ruhl, S. B. M. Kretschmer, B. Hofmann, B. Kuhn, J. Fettel, A. Vogel, K. T. Flugel, G. Manolikakes, N. Hellmuth, A. K. Hafner, V. Golghalyani, A. K. Ball, M. Piesche, C. Matrone, G. Geisslinger, M. J. Parnham, M. Karas, D. Steinhilber, J. Roos, and T. J. Maier, Biochem. Pharmacol., 125, 55 (2017).
K. Kim, S. J. Kim, Y. T. Han, S. J. Hong, H. An, D. J. Chang, T. Kim, B. Lim, J. Lee, Y. J. Surh, and Y. G. Suh, Bioorg. Med. Chem. Lett., 25, 5444 (2015).
X. Yu, Q. Zhang, L. Tian, Z. Guo, C. Liu, J. Chen, W. Ebrahim, Z. Liu, P. Proksch, and K. Zou, J. Nat. Prod., 81, 85 (2018).
J. Chun, R. J. Li, M. S. Cheng, and Y. S. Kim, Cancer Lett., 357, 393 (2015).
P. Y. Liu, D. Liu, and W. H. Li, Chem. Biodiv., 12, 1481 (2015).
H. Hendriks, Th. M. Malingre, and E. T. Elema, Pharm. Weekbl. Sci., 5, 281 (1983).
K. Ito, Y. Sakakibara, and M. Haruna, Phytochemistry, 21, 715 (1982).
S. P. Yang, J. Huo, Y. Wang, L. G. Lou, and J. M. Yue, J. Nat. Prod., 67, 638 (2004).
S. P. Yang, J. G. Cheng, J. Huo, H. L. Jiang, K. X. Chen, and J. M. Yue, Chin. J. Chem., 23, 1530 (2005).
F. Wang, H. H. Zhong, S. Q. Fang, Y. F. Zheng, C. Y. Li, G. P. Peng, and X. C. Shen, Planta Med., 84, 123 (2018).
M. G. Repetto and A. Boveris, Mini-Rev. Med. Chem., 10, 615 (2010).
Y. Ren, U. M. Acuna, F. Jimenez, R. Garcia, M. Mejia, H. Chai, J. C. Gallucci, N. R. Farnsworth, D. D. Soejarto, E. J. Carcache de Blanco, and A. D. Kinghorn, Tetrahedron, 68, 2671 (2012).
T. Itoh, M. Oyama, N. Takimoto, C. Kato, Y. Nozawa, Y. Akao, and M. Iinuma, Bioorg. Med. Chem., 17, 3189 (2009).
J. Tasqiah, H. Yoshie, Z. Stefanie, K. Marcel, H. Matthias, and A. Michael, Fitoterapia, 82, 955 (2011).
M. Chadwick, H. Trewin, F. Gawthrop, and C. Wagstaff, Int. J. Mol. Sci., 14, 12780 (2013).
M. Maas, A. Hensel, F. B. Costa, R. Brun, M. Kaiser, and T. J. Schmidt, Phytochemistry, 72, 635 (2011).
K. Ito, Y. Sakakibara, and M. Haruna, Chem. Lett., 8, 1473 (1979).
D. Youssef and A. W. Frahm, Planta Med., 60, 267 (1994).
Luis R. Hernandez, Cesar A. N. Catalan, Carlos M. Cerda-Garcia-Rojas, and P. Joseph-Nathan, Phytochemistry, 37, 1331 (1994).
F. Bohlmann, Nezhun Ates (Goren), J. Jakupovic, R. M. King, and H. Robinson, Phytochemistry, 23, 1180 (1984).
W. Herz, H. Grisebach, and G. W. Kirby (eds.), Progress in the Chemistry of Organic Natural Products, Vol. 38, Springer, Vienna, 1979, pp. 47–390.
J. Huo, S. P. Yang, J. Ding, and J. M. Yue, J. Nat. Prod., 67, 1470 (2004).
Acknowledgment
The work was financially supported by the National Natural Science Foundation of China (81773868), the Natural Science Foundation of Zhejiang Province (LY17H280004), the Zhejiang Province Chinese medicine scientific research fund project (2016ZA050), and the Hangzhou Major Science and Technology Project (20172016A01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2020, pp. 565–568.
Rights and permissions
About this article
Cite this article
Jiang, Ql., Shou, Pt., Sun, Mj. et al. A New Sesquiterpene Lactone from Eupatorium chinense and its Anti-TNBC Activity. Chem Nat Compd 56, 651–655 (2020). https://doi.org/10.1007/s10600-020-03114-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03114-y