The mRNA vaccine space is emerging as a promising alternative to traditional vaccine platforms, as mRNA vaccines can be manufactured quickly and tailored for a broad range of conditions. In clinical studies, mRNA vaccines targeting various infectious diseases and cancer were generally safe and well tolerated. Over the past few years, multiple mRNA vaccines have been refined and validated in studies of immunogenicity and efficacy. Four of the vaccine candidates currently in clinical trials to prevent COVID-19 (which is caused by SARS-CoV-2) are mRNA vaccines: mRNA-1273 (Moderna), BNT-162 (BioNTech), CVnCoV (CureVac), and LNP-nCoVsaRNA (Imperial College London).
To better understand the intellectual property (IP) surrounding mRNA vaccine development, we generated a detailed IP landscape by reviewing and indexing English-language US, European, and international (covered by the Patent Cooperation Treaty) patents and applications published from January 2010 to April 2020 (Supplementary information). Documents were limited to filings containing claims restricted to vaccines encoded by mRNA. We identified 113 INPADOC patent families relevant to the search focus that were indexed by indication, methods of delivery and pharmacological modifications based on the claims (Fig. 1).
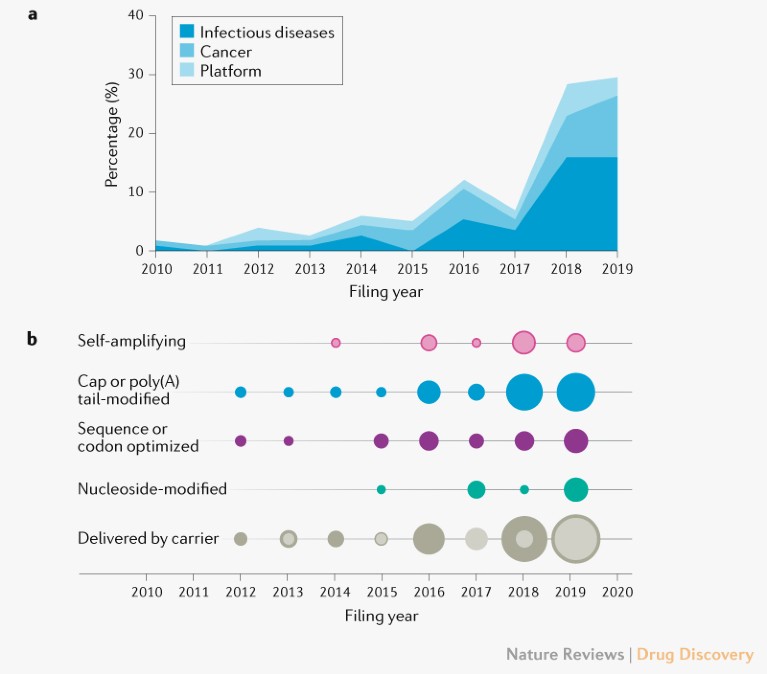
Fig. 1 | Patent filing activity for mRNA vaccine by indication focus and optimization methods. a | Filing activity plotted by filing year and indication focus (infectious diseases, cancer and platforms). b | Patent documents plotted by filing year and by optimization methods to address mRNA instability, inefficient in vivo delivery and innate immunogenicity. Circles are coloured according to optimization method and the area of the circle indicates the total number of patent or applications filed in a given year. The area in the concentric circles indicates the number of patents or applications filed in a given year claiming delivery by lipid nanoparticle.
The patent-filing activity grew dramatically over the past 5 years for both infectious disease and cancer indications. The number of applications for infectious disease indications surpassed those for cancer over the past 3 years, which could reflect increased interest in vaccines following epidemic outbreaks of MERS-CoV, Ebola virus and Zika virus. In August 2019, Moderna received FDA Fast Track Designation for an investigational Zika virus vaccine (mRNA-1893) currently being evaluated in a phase I study.
A patent landscape of assignees claiming an mRNA vaccine was generated from the collected set of documents. Roughly 70% of the patent families were filed by industry (80 out of 113). From a total of 56 assignees, nearly half (26) are companies and more than a third of those (10; 38%) are publicly traded. The remaining 54% (30) of the assignees are research institutions or independent inventors. The space is highly fragmented and includes multinational companies such as Bayer, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline (GSK) and Hisamitsu Pharmaceutical. GSK is currently testing a self-amplifying mRNA vaccine for rabies with a built-in adjuvant in a phase I clinical trial. Smaller companies, such as Morphogenesis, Multivir, Nantbio and Translate Bio (formerly RaNA Therapeutics), each have a small IP portfolio on mRNA vaccines focused on cancer treatment.
Moderna, CureVac, BioNTech and GSK collectively own nearly half of the mRNA vaccine patent applications. Moderna started a phase III clinical trial of their SARS-CoV-2 mRNA vaccine in July, and have presented positive phase I data for nine prophylactic vaccines, including those for cytomegalovirus and Zika virus. BioNTech, in collaboration with Pfizer, is examining four vaccines against SARS-CoV-2 in phase I/II clinical trials and initiated a phase II/III clinical trial of one of these, BNT162b2, in July. CureVac initiated a phase I/IIa clinical trial with its lead SARS-CoV-2 mRNA vaccine candidate in June, and Imperial College London also initiated a phase I clinical trial of their self-amplifying RNA vaccine in June.
Overall filing activity indicates an increasing number of documents with claims to protect methods to improve mRNA delivery efficiency for mRNA that is delivered by a carrier, namely lipid nanoparticle (LNP) compositions. Numerous patents protect pharmacological modifications to reduce mRNA instability and innate immunogenicity (Fig. 1b). We anticipate that the space will grow exponentially as result of increased investment in mRNA vaccine platforms and the ongoing, accelerated clinical trials, which are potentially proof of concept for mRNA vaccines. Positive results in these trials would not only solve an immediate urgent need for a vaccine against SARS-CoV-2, but also provide a potent and versatile therapeutic tool for future outbreaks of infectious diseases.
