Abstract
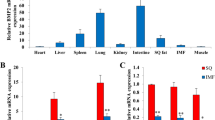
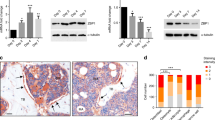
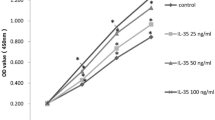
Our previous study revealed that 3T3-L1 preadipocytes can differentiate to either osteoblasts or adipocytes in response to bone morphogenic protein 9 (BMP9). In the present study, we try to further investigate whether the Wnt/β-catenin signaling plays a crucial role in this process. It was found that BMP9 effectively activated the Wnt/β-catenin signaling, and induced the expression levels of certain canonical Wnt ligands and their receptors in preadipocytes. Exogenous expression of β-catenin, Wnt1, Wnt3a, and Wnt10b potentiated BMP9-induced alkaline phosphatase (ALP) activity, while β-catenin knockdown or Dickkopf 1 (Dkk1) diminished BMP9-induced ALP activity. Moreover, it was demonstrated that β-catenin overexpression promoted BMP9-induced mineralization, and increased the expression levels of late osteogenic markers osteopontin and osteocalcin. Furthermore, β-catenin inhibited BMP9-induced lipid accumulation and the adipogenic marker adipocyte fatty acid binding protein (aP2). The cell-implantation assay results identified that β-catenin not only augmented BMP9-induced ectopic bone formation, but also blocked adipogenesis in vivo. Mechanistically, it was found that β-catenin and BMP9 synergistically stimulated the osteogenic transcription factors runt-related transcription factor 2 (Runx2) and Osterix (OSX). However, BMP9-induced adipogenic transcription factors, peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer-binding protein α (C/EBPα), were inhibited by β-catenin. Therefore, these findings suggested that the Wnt/β-catenin signaling, potentially via the modulation of osteogenic and adipogenic transcriptional factors, exerts an opposite effect on BMP9-induced osteogenic and adipogenic differentiation in preadipocytes.





Similar content being viewed by others
References
Meunier, P., Aaron, J., Edouard, C., & Vignon, G. (1971). Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clinical Orthopaedics and Related Research, 80, 147–154. https://doi.org/10.1097/00003086-197110000-00021.
Verma, S., Rajaratnam, J. H., Denton, J., Hoyland, J. A., & Byers, R. J. (2002). Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. Journal of Clinical Pathology, 55, 693–698. https://doi.org/10.1136/jcp.55.9.693.
Justesen, J., Stenderup, K., Ebbesen, E. N., Li, M., Steiniche, T., & Kassem, M. (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology, 2, 165–171. https://doi.org/10.1023/A:1011513223894.
Justesen, J., Stenderup, K., Eriksen, E. F., Kassem, M., Justesen, J., Stenderup, K., Eriksen, E. F., & Kassem, M. (2002). Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcified Tissue International, 71, 36–44. https://doi.org/10.1007/s00223-001-2059-x.
Gimble, J. M., Zvonic, S., Floyd, Z. E., Kassem, M., & Nuttall, M. E. (2006). Playing with bone and fat. Journal of Cellular Biochemistry, 98, 251. https://doi.org/10.1002/jcb.20777.
Park, S. R., Oreffo, R. O., & Triffitt, J. T. (1999). Interconversion potential of cloned human marrow adipocytes in vitro. Bone, 24, 549. https://doi.org/10.1016/s8756-3282(99)00084-8.
Justesen, J., Pedersen, S. B., Stenderup, K., & Kassem, M. (2004). Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Engineering, 10, 381–391. https://doi.org/10.1089/107632704323061744.
Park, J. G., Lee, D. H., Moon, Y. S., & Kim, K. H. (2014). Reversine increases the plasticity of lineage-committed preadipocytes to osteogenesis by inhibiting adipogenesis through induction of TGF-β pathway in vitro. Biochemical & Biophysical Research Communications, 446, 30–36. https://doi.org/10.1016/j.bbrc.2014.02.036.
Skillington, J., Choy, L., & Derynck, R. (2002). Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. Journal of Cell Biology, 159, 135–146. https://doi.org/10.1083/jcb.200204060.
MacDonald, B. T., Tamai, K., & Xi, H. (2009). Wnt/β-catenin signaling: components, mechanisms, and diseases. Developmental Cell, 17, 0–26. https://doi.org/10.1016/j.devcel.2009.06.016.
Congdon, K. L., Voermans, C., Ferguson, E. C., Dimascio, L. N., & Reya, T. (2008). Activation of Wnt signaling in hematopoietic regeneration. Stem Cells, 26, 1202–1210. https://doi.org/10.1634/stemcells.2007-0768.
Zhan, T., Rindtorff, N., & Boutros, M. (2016). Wnt signaling in cancer. Oncogene, 36, 1461–1473. https://doi.org/10.1038/onc.2016.304.
Nd, G. D., & Karsenty, G. (2007). In vivo analysis of Wnt signaling in bone. Endocrinology, 148, 2630–2634. https://doi.org/10.1210/en.2006-1372.
Cadigan, K. M., & Nusse, R. (1997). Wnt signaling: a common theme in animal development. Genes Development, 11, 3286–3305. https://doi.org/10.1101/gad.11.24.3286.
Karsenty, G., & Wagner, E. F. (2002). Reaching a genetic and molecular understanding of skeletal development. Developmental Cell, 2, 389–406. https://doi.org/10.1016/S1534-5807(02)00157-0.
Kim, J. H., Liu, X., Wang, J., Chen, X., Zhang, H., Kim, S. H., Cui, J., Li, R., Zhang, W., & Kong, Y. (2013). Wnt signaling in bone formation and its therapeutic potential for bone diseases. Therapeutic Advances in Musculoskelet Disease, 5, 13–31. https://doi.org/10.1177/1759720X12466608.
Liu, Y., Liu, Y. Y., Zhang, R. X., Wang, X., Huang, F., Yan, Z. J., Mao, N., Huang, J., Wang, Y. Z., Wang, Y., Chen, L., Yin, L. J., He, B. C., & Deng, Z. L. (2014). All-trans retinoic acid modulates bone morphogenic protein 9-induced osteogenesis and adipogenesis of preadipocytes through BMP/Smad and Wnt/β-catenin signaling pathways. International Journal of Biochemistry & Cell Biology, 47, 47–56. https://doi.org/10.1016/j.biocel.2013.11.018.
Tang, N., Song, W. X., Luo, J., Luo, X., Jin, C., Sharff, K. A., Yang, B., He, B. C., Huang, J. Y., & Zhu, G. H. (2009). BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. Journal of Cellular & Molecular Medicine, 13, 2448–2464. https://doi.org/10.1111/j.1582-4934.2008.00569.x.
Pi, C. J., Liang, K. L., Ke, Z. Y., Chen, F., Cheng, Y., Yin, L. J., Deng, Z. L., He, B. C., & Chen, L. (2016). Adenovirus-mediated expression of vascularendothelial growth factor-a potentiates bone morphogenetic protein 9-induced osteogenic differentiation and bone formation. Biological Chemistry, 397, 765–775. https://doi.org/10.1515/hsz-2015-0296.
Lin, L., Qiu, Q., Zhou, N., Dong, W., Shen, J., Jiang, W., Fang, J., Hao, J., & Hu, Z. (2016). Dickkopf-1 is involved in BMP9-induced osteoblast differentiation of C3H10T1/2 mesenchymal stem cells. BMB Reports, 49, 179–184. https://doi.org/10.5483/BMBRep.2016.49.3.206.
Chen, Y., Whetstone, H. C., Youn, A., Nadesan, P., Chow, E. C., Lin, A. C., & Alman, B. A. (2007). Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. Journal of Biological Chemistry, 282, 526–533. https://doi.org/10.1074/jbc.m602700200.
Papathanasiou, I., Malizos, K. N., & Tsezou, A. (2012). Bone morphogenetic protein-2-induced Wnt/β-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes.Arthritis Research & Therapy, 14(2), 1–14. https://doi.org/10.1186/ar3805.
Zhang, M., Yan, Y., Lim, Y. B., Tang, D., Xie, R., Chen, A., Tai, P., Harris, S. E., Xing, L., & Qin, Y. X. (2009). BMP-2 modulates β-catenin signaling through stimulation of Lrp5 expression and inhibition of β-TrCP expression in osteoblasts. Journal of Cellular Biochemistry, 108, 896–905. https://doi.org/10.1002/jcb.22319.
Yang, L., Yamasaki, K., Shirakata, Y., Dai, X., Tokumaru, S., Yahata, Y., Tohyama, M., Hanakawa, Y., Sayama, K., & Hashimoto, K. (2006). Bone morphogenetic protein-2 modulates Wnt and frizzled expression and enhances the canonical pathway of Wnt signaling in normal keratinocytes. Journal of Dermatological Science, 42, 111–119. https://doi.org/10.1016/j.jdermsci.2005.12.011.
Kim, H. K. W., Oxendine, I., & Kamiya, N. (2013). High-concentration of BMP2 reduces cell proliferation and increases apoptosis via DKK1 and SOST in human primary periosteal cells. Bone, 54, 141–150. https://doi.org/10.1016/j.bone.2013.01.031.
Kamiya, N., Kobayashi, T., Mochida, Y., Yu, P. B., Yamauchi, M., Kronenberg, H. M., & Mishina, Y. (2010). Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. Journal of Bone & Mineral Research, 25, 200–210. https://doi.org/10.1359/jbmr.090806.
Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127, 469–480. https://doi.org/10.1016/j.cell.2006.10.018.
Zhu, J. H., Liao, Y. P., Li, F. S., Hu, Y., Li, Q., Ma, Y., Wang, H., Zhou, Y., He, B. C., & Su, Y. X. (2018). Wnt11 promotes BMP9-induced osteogenic differentiation through BMPs/Smads and p38 MAPK in mesenchymal stem cells. Journal of Cellular Biochemistry, 119, 9462–9473. https://doi.org/10.1002/jcb.27262.
Gong, Y., Slee, R. B., Fukai, N., Rawadi, G., Romanroman, S., Reginato, A. M., Wang, H., Cundy, T., Glorieux, F. H., & Lev, D. (2001). LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell, 107, 513–523. https://doi.org/10.1016/S0092-8674(01)00571-2.
Georges, R., Béatrice, V., Fred, D., Roland, B., & Sergio, R. R. (2010). BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. Journal of Bone & Mineral Research, 18, 1842–1853. https://doi.org/10.1359/jbmr.2003.18.10.1842.
Zhang, H., Wang, J., Deng, F., Huang, E., Yan, Z., Wang, Z., Deng, Y., Zhang, Q., Zhang, Z., & Ye, J. (2015). Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials, 39, 145–154. https://doi.org/10.1016/j.biomaterials.2014.11.007.
Shen, J., James, A. W., Zhang, X., Shen, P., Zara, J. N., Asatrian, G., Chiang, M., Min, L., Khadarian, K., & Nguyen, A. (2016). Novel Wnt regulator NEL-Like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. American Journal of Pathology, 186, 419–434. https://doi.org/10.1016/j.ajpath.2015.10.011.
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A. L., & Karsenty, G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell, 89, 747–754. https://doi.org/10.1016/s0092-8674(00)80257-3.
Tripti, G., Lengner, C. J., Hayk, H., Bhat, R. A., Bodine, P. V. N., Komm, B. S., Amjad, J., Wijnen, A. J. V., Stein, J. L., & Stein, G. S. (2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. Journal of Biological Chemistry, 280, 33132–33140. https://doi.org/10.1074/jbc.M500608200.
Takahashi, T. (2011). Overexpression of Runx2 and MKP-1 stimulates transdifferentiation of 3T3-L1 preadipocytes into bone-forming osteoblasts in vitro. Calcified Tissue International, 88, 336–347. https://doi.org/10.1007/s00223-011-9461-9.
Zhang, Y., Li, X., Qian, S., Guo, L., Huang, H., He, Q., Liu, Y., Ma, C., & Tang, Q. Q. (2012). Down-regulation of Type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Molecular Endocrinology, 26, 798–808.
Enomoto, H., Furuichi, T. A., Yamana, K., Yoshida, C., Sumitani, S., Yamamoto, H., Enomoto-Iwamoto, M., Iwamoto, M., & Komori, T. (2004). Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. Journal of Cell Science, 117, 417 https://doi.org/10.1242/jcs.00866.
Gori, F., Thomas, T., Hicok, K. C., Spelsberg, T. C., & Riggs, B. L. (2010). Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. Journal of Bone & Mineral Research, 14, 1522–1535. https://doi.org/10.1359/jbmr.1999.14.9.1522.
Kang, S., Bennett, C. N., Gerin, I., Rapp, L. A., & Macdougald, O. A. (2007). WNT signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing c/EBPα and PPARγ. Journal of Biological Chemistry, 282, 14515–14524. https://doi.org/10.1074/jbc.m700030200.
Acknowledgements
We thank Dr. TC He (University of Chicago Medical Center, USA) for providing recombinant adenoviruses and pTOP-luc reporter plasmid. The present study was supported by Natural Science Foundation of China (grant no. 81601895).
Author information
Authors and Affiliations
Contributions
Y.L., K.L.L., and L.C. conceived and designed the study. K.L.L., R.D.L., Z.J.Y., and L.Y.W. performed the experiments. Y.L. and K.L.L. collected and analyzed the data. Y.L. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, K., Du, Y., Chen, L. et al. Contrary Roles of Wnt/β-Catenin Signaling in BMP9-Induced Osteogenic and Adipogenic Differentiation of 3T3-L1 Preadipocytes. Cell Biochem Biophys 78, 347–356 (2020). https://doi.org/10.1007/s12013-020-00935-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-020-00935-0