Abstract
The amount of structural and storage lipids and the composition of fatty acids (FA) in the symbiotic hydroid coral Millepora dichotoma were determined monthly throughout the year. In the first half of the year, the hydrocoral actively accumulated lipids, as indicated by a twofold increase in the content of reserve lipid classes of (waxes, triglycerides, and monoalkyl diacyl glycerides) concurrent with a relatively stable level of polar phospholipids (PL) and sterols. There was a decrease in the proportion between choline and ethanolamine glycerophospholipids (PC/PE) in July, a sharp drop in the content of triglycerides in August, and a large dispersion the PL values in September, which may be due to energy expenditures and structural changes in the colonies during spawning. The level of 22:6n-3, which is synthesized in hydrocoral from FA of plankton, was maximum in the summer, and the level of 18:4n-3, the FA marker of photosynthetic symbionts, doubled in the winter. This indicates the predominance of the heterotrophic mode of nutrition in M. dichotoma in the summer and an increased role of symbionts (an autotrophic food source) in the winter with a drop in water temperature and the onset of a stormy period.
Similar content being viewed by others
INTRODUCTION
The structural basis of the tropical coral reef ecosystem, one of the most productive shallow marine ecosystems, consists of corals (Cnidaria: Anthozoa) and hydrocorals (Cnidaria: Hydrozoa) [1]. Soft tissue of polyps of these colonial animals, surrounded by a hard exoskeleton, are rich in lipids. Most of total lipid (TL) are neutral lipid classes (esters of aliphatic alcohols and fatty acids or waxes (WX), triacylglycerols (TAG) and monoalkyl diacylglycerols (MADAG)) [2], which serve as an energy reserve for cnidarians. Approximately one third of the mass of coral and hydrocoral TL are sterols (ST) and polar lipids (PL), primarily phosphorus-containing lipids (PhL) [3], which are the structural basis of cell membranes. The level of TL is used to assess the health of colonies [4], food strategy [5], the influence of environmental factors [6, 7], the reproductive strategy of coral [9–11], as well as to study the processes of bleaching and recovery of coral reefs [12, 13]. As a rule, to evaluate the energy balance of corals, only the amount of TL is determined, despite the fundamental differences in the functions of storage and structural lipid classes. Moreover, all studies on the dynamics of the content and composition of TL were performed on various species of corals, while similar research on hydrocorals is lacking.
Several studies have described the composition of TL, PhL, and fatty acids (FA) of hydrocorals of the genus Millepora [3, 14–16], which, like reef-building corals, harbor intracellular symbiotic microalgae (zooxanthellae) [1]. Seasonal changes in the lipid composition in Millepora have not been examined. In this study, we, for the first time, performed a monthly analysis of the content of WX, TAG, MADAG, ST, PL, classes of PhL, and FA composition in colonies of the hydrocoral Millepora dichotoma collected over one year on a coral reef in the coastal waters of Vietnam. The data on seasonal lipid changes could be useful for assessing the role of PL in total lipid changes, the stability of the PhL composition of cell membranes, and the contribution of heterotrophy and autotrophy to feeding of tropical hydrocorals.
MATERIALS AND METHODS
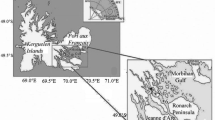
Colonies of the hydroid coral Millepora dichotoma Forskål, 1775 (Cnidaria: Hydrozoa: Anthoathecata: Milleporidae) were collected at a depth of 2–4 m in Nha Trang Bay, the South China Sea (12°17′ N, 109°14′ E), from January to December 2018. Seven colonies were collected each month. Polyps were washed from each colony with water under pressure, suspended, and divided into two equal parts. One part of the suspension was dried at 150°C to a constant dry weight (dr.w.). Total lipids from the second part of the suspension were extracted with a mixture of chloroform and methanol, as described previously [17]. TL of each colony was evaporated under reduced pressure, weighed, dissolved in chloroform, and stored at –80°С.
The composition of TL and PhL was analyzed by thin layer chromatography (TLC) on silica gel, followed by densitometry, as described previously [18]. For the PhL analysis, the system of chloroform : methanol : 28% ammonia, 65 : 35 : 5 (v/v/v), was used. For the analysis of TL, the plate was eluted to the full length with a system of n-hexane : diethyl ether : acetic acid, 70 : 30 : 1 (v/v/v), dried, and re-eluted to 25% length with a system of chloroform : methanol : 28% ammonia, 65 : 35 : 5 (v/v/v).
The composition of FA was analyzed by gas chromatography [19] on an Equity-5 capillary column (30 m × 0.25 mm) in the form of FA methyl esters obtained by acidic methanolysis of TL. The structure of FA was determined in the form of 4,4-dimethyloxazoline (DMOX) derivatives on an MDN-5s capillary column (30 m × 0.25 mm) using a mass spectrometric detector.
Statistical analysis of the data was carried out using the Statistica 8.0 software package (StatSoft, Inc., USA). A significance level of 5% was used when comparing the average values of the content of lipid classes and FA (one-way ANOVA, post-hoc Tukey HSD test). Values are presented as mean ± standard deviation (n = 7).
RESULTS
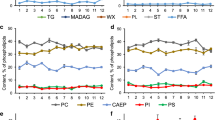
The amount of TL in the polyps of M. dichotoma varied from the minimum values in November–March (January: 71.9 ± 22.4 mg/g dr.w.) to the maximum in the summer (June: 150.0 ± 76.4 mg/g dr.w.). In TL, the main classes of structural lipids were PL and ST, and the main storage lipid classes were WX, TAG, and MADAG (Table 1). Among the minor components, free FA were present; their content did not significantly change during the year (2.1 ± 0.6 mg/g dr.w.).
In December–January, the amount of PL and TAG in M. dichotoma tissues was significantly higher than the amount of WX and MADAG. In June–July, WX, TAG, and MADAG prevailed among TL. The annual dynamics of the content of structural lipids sharply differed from the dynamics of the content of storage lipid classes (Table 1). During the year, the average content of PL (21.7 ± 9.7 mg/g dr.w.) and ST (9.7 ± 1.9 mg/g dr.w.) remained relatively stable, with the exception of a decrease in March (12.6 ± 2.3 and 5.3 ± 0.6 mg/g dr.w., respectively) (HSD test, P < 0.05). On the contrary, the dependences of the amount of WX, TAG, and MADAG on the season had a distinct maximum in the summer and a minimum in the winter (HSD test, P <0.05); the maximum and minimum contents of WX, TAG, and MADAG differed by factors of 3.5, 2.3, and 2.9, respectively. There was a sharp decrease in the TAG content in August–September (25.4 ± 2.1 mg/g dr.w.), compared to the maximum in June (40.8 ± 4.9 mg/g dr.w.), as well as a large dispersion of the PL content in September (30.8 ± 10.1 mg/g dr.w.).
Four classes of phospholipids were identified in PhL, namely, ethanolamine glycerophospholipids (PE), choline glycerophospholipids (PC), serine glycerophospholipids (PS), and inositol glycerophospholipids (PI), as well as a phosphonolipid ceramide aminoethyl phosphonate (CAEP). During a year, the average levels of PS, PI, and CAEP (4.9 ± 0.9, 4.2 ± 0.7, and 20.0 ± 1.9% of total PhL, respectively) remained virtually unchanged (P > 0.05). In the first half of the year, the level of PC was significantly higher (HSD test, P < 0.05) than the level of PE (38.7 ± 1.6 and 32.0 ± 2.0% of the total PhL, respectively, Table 1). Since July, the concentration of PC and PE did not differ significantly (P > 0.05).
Saturated (14:0, 16:0, 18:0, and 20:0), monounsaturated (16:1n-7, 18:1n-9, 18:1n-7, 20:1n-9, and 22:1), and polyunsaturated acids (18:2n-6, 18:3n-6, 18:3n-3, 18:4n-3, 20:4n-6, 20:4n-3, 20:5n-3, 22:4n-6, 22:5n-6, 22:5n-3, and 22:6n-3) were found in total FA obtained by hydrolysis of TL from M. dichotoma. The proportion of docosahexaenoic (22:6n-3) and palmitic (16:0) acids in the total FA was the largest in all samples throughout the year (Table 1). The average percentage of 22:6n-3, very high in the 1st–3rd quarters of the year (about 30%), markedly decreased in the 4th quarter (17.6 ± 6.4%) (HSD test, P < 0.05). The level of 16:0 did not significantly change (P > 0.05), while the highest level of 18:4n-3 was recorded in the winter, in December (8.2 ± 2.4%) (HSD test, P < 0.05), against the minimum values in the period from March to October (4–5% of the total FA) (Table 1). The content of 22:5n-6 amounted to 8–10% of the total FA and practically did not change during the year.
DISCUSSION
It is known that annual changes in water temperature and solar radiation cause cyclical changes in the coral reef ecosystem. In Nha Trang Bay, water temperature fluctuates between 28 and 30°С most of the year (April–September) and drops to 24–25°С in December–January, while the maximum solar radiation is observed between January and April [20]. In addition, monsoon storms from October to December greatly increase turbulence and turbidity in shallow areas of the reef.
Similar to lipids of reef-building corals [7], the TL content in M. dichotoma tissues was maximum in the summer, minimum in the winter, and correlated with water temperature (r = 0.901). Structural and storage lipids were found to make different contributions to the TL dynamics. The annual change in the amount of TL was caused by a sharp change in the contents of storage lipids against the background of the relatively constant level of structural lipids (PL and ST). The 1.5‑fold change in the amount of TL was accompanied by a 2–3-fold change in the amount of WX, TAG, and MADAG. Using M. dichotoma as an example, it can be seen that the level of storage lipids may be a more sensitive criterion than the level of TL. In addition, the level of PL and ST in cnidarians is species-specific [2, 3, 21]; therefore, different initial levels of structural lipids can distort the results of energy demands in different types of cnidarians, when made only on the basis of the TL dynamics [22].
The number and composition of coral PL and PhL are very sensitive to rapid changes in environmental parameters. For example, when seawater is warmed above 32°C, which causes the loss of zooxanthellae and the death of coral reefs, the PL level in symbiotic species of cnidarians changes noticeably [12, 23, 24]. Under a short-term temperature stress, the composition of coral PhL classes changes dramatically [25]. Our study demonstrates that the annual cycle has a little effect on the composition of PhL in M. dichotoma tissues. This indicates the stability of the structure of cell membranes of hydrocoral under a slow, natural change in its environment.
Sexual reproduction of Millepora is seasonal; it begins with the appearance of ampullae and ends with the release of planktonic medusae in April–May in Taiwan, from April to July in Barbados, and from June to March in Curaçao [1]. There are no data on lipids of Millepora reproductive materials; however, several reports on the role of lipids in the reproduction of corals and other cnidarians indicate that their reproductive materials are very rich in lipids, most of which are WX or TAG [26, 27]. The maturation and release of planktonic medusae should be accompanied by energy expenditures [10] and a mechanical loss of reserve lipids, especially WX and TAG, as well as by the lability of the composition and amount of membrane PL during the formation/opening of ampullae and the subsequent regeneration of the colony. We assume that the change in the PE/PC ratio in July, the gap in the level of TAG and WX in August, as well as the large dispersion of the PL content in September, may accompany the process of maturation and spawning of M. dichotoma.
The composition of FA is widely used to determine changes in the composition of food sources of corals [5, 28]. Compared to lipids of corals and cold-water hydroid coral species, Millepora lipids have a very low level of 20:4n-6 and 20:5n-3, a very high content of 22:6n-3, and contain a rare 22:5n-6 acid [3, 21]. We suppose that this specific FA profile is the result of the high activity of C2 elongase and Δ4 desaturase, which convert 20:5n-3 to 22:6n-3 and 20:4n-6 to 22:5n-6 [16]. Plankton, one of the main food sources for cnidarians, is a source of 20:5n-3 for biosynthesis of 22:6n-3 [29]. A noticeable increase in the level of 22:6n-3 in M. dichotoma during the summer can be due to the increased contribution of plankton to hydrocoral food as a consequence of enhanced plankton production with a seasonal increase in water temperature and solar radiation. In October–December, stormy conditions, combined with a decrease in water temperature, can cause a decrease in the intake of 20:5n-3 from food and a decrease in the level of 22:6n-3 in M. dichotoma lipids. When the availability of external food sources is reducing, the importance of zooxanthellae, which produce organic substances through photosynthesis and transfer them to the host [30], is rising for M. dichotoma. The 18:4n-3 acid is the marker of zooxanthellae [8], and the level of 18:4n-3 reflects the amount of intracellular symbiotic microalgae [24]. In the cold period, the level of 18:4n-3 in the hydrocoral doubled, which indicates an increase in the number and/or productivity of zooxanthellae. We suppose that the heterotrophic mode of nutrition predominates in M. dichotoma in the summer period, and the role of the autotrophic food source increases in the winter. Thus, the study of the annual dynamics of polar and storage lipids, as well as their FA markers, provides new information on the dynamics of the energy budget, reproduction, and nutrition of hydroid corals Millepora.
REFERENCES
Lewis, J.B., Biology and ecology of the hydrocoral Millepora on coral reefs, Adv. Mar. Biol., 2006, vol. 50, pp. 1–55.
Imbs, A.B., Fatty acids and other lipids of corals: Composition, distribution, and biosynthesis, Russ. J. Mar. Biol., 2013, vol. 39, pp. 153–168. https://doi.org/10.1134/s1063074013030061
Imbs, A.B., Latyshev, N.A., Dautova, T.N., and Latypov, Yu.Ya., Distribution of lipids and fatty acids in corals by their taxonomic position and presence of zooxanthellae, Mar. Ecol.: Prog. Ser., 2010, vol. 409, pp. 65–75. https://doi.org/10.3354/meps08622
Yamashiro, H., Oku, H., Onaga, K., et al., Coral tumors store reduced level of lipids, J. Exp. Mar. Biol. Ecol., 2001, vol. 265, pp. 171–179. https://doi.org/10.1016/S0022-0981(01)00333-1
Seemann, J., Sawall, Y., Auel, H., and Richter, C., The use of lipids and fatty acids to measure the trophic plasticity of the coral Stylophora subseriata,Lipids, 2013, vol. 48, pp. 275–286. https://doi.org/10.1007/s11745-012-3747-1
Ben-David-Zasow, R. and Benayahu, Y., Temporal variation in lipid, protein and carbohydrate content in the Red Sea soft coral Heteroxenia fuscescens,J. Mar. Biol. Assoc. U. K., 1999, vol. 79, pp. 1001–1006.
Oku, H., Yamashiro, H., Onaga, K., et al., Seasonal changes in the content and composition of lipids in the coral Goniastrea aspera,Coral Reefs, 2003, vol. 22, pp. 83–85.
Bishop, D.G. and Kenrick, J.R., Fatty acid composition of symbiotic zooxanthellae in relation to their hosts, Lipids, 1980, vol. 15, pp. 799–804.
Ward, S., Two patterns of energy allocation for growth, reproduction and lipid storage in the scleractinian coral Pocillopora damicornis,Coral Reefs, 1995, vol. 14, pp. 87–90.
Viladrich, N., Bramanti, L., Tsounis, G., et al., Variation in lipid and free fatty acid content during spawning in two temperate octocorals with different reproductive strategies: surface versus internal brooder, Coral Reefs, 2016, vol. 35, pp. 1033–1045. https://doi.org/10.1007/s00338-016-1440-1
Grinyó, J., Viladrich, N., Diaz, D., et al., Reproduction, energy storage and metabolic requirements in a mesophotic population of the gorgonian Paramuricea macrospina, PLoS One, 2018, vol. 13, art. ID e0203308. https://doi.org/10.1371/journal.pone.0203308
Yamashiro, H., Oku, H., and Onaga, K., Effect of bleaching on lipid content and composition of Okinawan corals, Fish. Sci., 2005, vol. 71, pp. 448–453. https://doi.org/10.1111/j.1444-2906.2005.00983.x
Wall, C.B., Ritson-Williams, R., Popp, B.N., and Gates, R.D., Spatial variation in the biochemical and isotopic composition of corals during bleaching and recovery, Limnol. Oceanogr., 2019, vol. 64, pp. 2011–2028. https://doi.org/10.1002/lno.11166
Latyshev, N.A., Naumenko, N.V., Svetashev, V.I., and Latypov, Y.Ya., Fatty acids of reef-building corals, Mar. Ecol.: Prog. Ser., 1991, vol. 76, pp. 295–301.
Yamashiro, H., Oku, H., Higa, H., et al., Composition of lipids, fatty acids and sterols in Okinawan corals, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1999, vol. 122, pp. 397–407. https://doi.org/10.1016/S0305-0491(99)00014-0
Imbs, A.B., Dang, L.P.T., and Nguyen, K.B., Comparative lipidomic analysis of phospholipids of hydrocorals and corals from tropical and cold-water regions, PLoS One, 2019, vol. 14, art. ID e0215759. https://doi.org/10.1371/journal.pone.0215759
Folch, J.F., Lees, M., and Sloane Stanley, G.H., A simple method for the isolation and purification of total lipids from animal tissue, J. Biol. Chem., 1957, vol. 226, pp. 497–509.
Imbs, A.B., Dang, L.P.T., Rybin, V.G., and Svetashev, V.I., Fatty acid, lipid class, and phospholipid molecular species composition of the soft coral Xenia sp. (Nha Trang Bay, the South China Sea, Vietnam), Lipids, 2015, vol. 60, pp. 575–589. https://doi.org/10.1007/s11745-015-4021-0
Imbs, A.B. and Grigorchuk, V.P., Lipidomic study of the influence of dietary fatty acids on structural lipids of cold-water nudibranch mollusk, Sci. Rep., 2019, vol. 9, art. ID 20013. https://doi.org/10.1038/s41598-019-56746-8
Nha Trang Sea Temperature, Global Sea Temperature. https://www.seatemperature.org/asia/vietnam/nha-trang.htm. Accessed February 10, 2020.
Imbs, A.B., Demidkova, D.A., and Dautova, T.N., Lipids and fatty acids of cold-water soft corals and hydrocorals: a comparison with tropical species and implications for coral nutrition, Mar. Biol., 2016, vol. 163, art. ID 202. https://doi.org/10.1007/s00227-016-2974-z
Levas, S., Schoepf, V., Warner, M.E., et al., Long-term recovery of Caribbean corals from bleaching, J. Exp. Mar. Biol. Ecol., 2018, vol. 506, pp. 124–134. https://doi.org/10.1016/j.jembe.2018.06.003
Rodrigues, L.J., Grottoli, A.G., and Pease, T.K., Lipid class composition of bleached and recovering Porites compressa Dana, 1846 and Montipora capitata Dana, 1846 corals from Hawaii, J. Exp. Mar. Biol. Ecol., 2008, vol. 358, pp. 136–143. https://doi.org/10.1016/j.jembe.2008.02.004
Imbs, A.B. and Yakovleva, I.M., Dynamics of lipid and fatty acid composition of shallow-water corals under thermal stress: an experimental approach, Coral Reefs, 2012, vol. 31, pp. 41–53. https://doi.org/10.1007/s00338-011-0817-4
Sikorskaya, T.V., Ermolenko, E.V., and Imbs, A.B., Effect of experimental thermal stress on lipidomes of the soft coral Sinularia sp. and its symbiotic dinoflagellates, J. Exp. Mar. Biol. Ecol., 2020, vol. 524, art. ID 151295. https://doi.org/10.1016/j.jembe.2019.151295
Arai, T., Kato, M., Heyward, A., et al., Lipid composition of positively buoyant eggs of reef building corals, Coral Reefs, 1993, vol. 12, pp. 71–75.
Figueiredo, J., Baird, A.H., Cohen, M.F., et al., Ontogenetic change in the lipid and fatty acid composition of scleractinian coral larvae, Coral Reefs, 2012, vol. 31, pp. 613–619. https://doi.org/10.1007/s00338-012-0874-3
Rocker, M.M., Francis, D.S., Fabricius, K.E., et al., Temporal and spatial variation in fatty acid composition in Acropora tenuis corals along water quality gradients on the Great Barrier Reef, Australia, Coral Reefs, 2019, vol. 38, pp. 215–228. https://doi.org/10.1007/s00338-019-01768-x
Dalsgaard, J., St. John, M., Kattner, G., et al., Fatty acid trophic markers in the pelagic marine environment, Adv. Mar. Biol., 2003, vol. 46, pp. 225–340. https://doi.org/10.1016/S0065-2881(03)46005-7
Stambler, N., The zooxanthellae–hard-coral symbiosis, in The Algal and Cyanobacteria Symbioses, New Jersey: World Scientific, 2016, pp. 423–466.
ACKNOWLEDGMENTS
The authors are grateful to D.S. Dat (Institute of Oceanography, Vietnam Academy of Science and Technology, Vietnam Academy of Science and Technology) for the collection of biological material and to B.S. Hoang (Institute of Oceanography, Vietnam Academy of Science and Technology) for the species identification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
FUNDING
This work was supported by the Russian Foundation for Basic Research (grant no. 19-54-54002) and the Vietnam Academy of Science and Technology (grant nos. QTRU01.05/19-20 and QTRU04.05/18-19).
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Imbs, A.B., Dang, L.T., Nguyen, K.B. et al. Annual Dynamics of the Composition of Polar Lipids, Storage Lipids, and Fatty Acid Markers in the Hydrocoral Millepora dichotoma Forskål, 1775 from Coastal Waters of Vietnam. Russ J Mar Biol 46, 221–225 (2020). https://doi.org/10.1134/S1063074020030062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074020030062