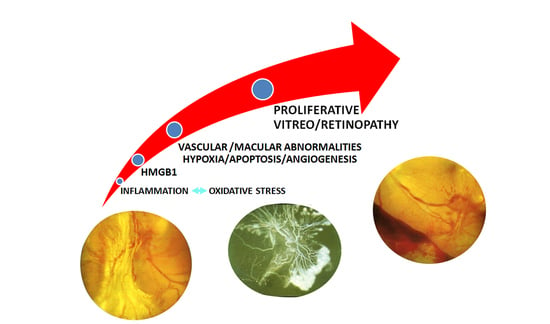
The Complex Relationship between Diabetic Retinopathy and High-Mobility Group Box: A Review of Molecular Pathways and Therapeutic Strategies
Abstract
:1. Introduction to Diabetic Retinopathy (DR)
2. Introduction to High-Mobility Group Box 1 (HMGB1)
3. HMGB1 and DR
4. Future Therapeutic Approaches
4.1. Glycyrrhizin (GA)
4.2. Small Interfering RNAs/Short Hairpin RNA (siRNA/shRNA)
4.3. Polygonum Cuspidatum (PCE)
4.4. Paeoniflorin
4.5. Salicin
4.6. Ethyl Pyruvate (EP)
4.7. Bradykinin (BK)
4.8. Kallistatin
4.9. Compound 49b
4.10. Cyclosporine A (CyA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxydeoxyguanosine |
| AGE | advanced glycation end-products |
| ARPE-19 | adult retinal pigment epithelial cell line-19 |
| BDNF | brain-derived neurotrophic factor |
| BK | bradykinin |
| BRB | blood–retinal barrier |
| COX-2 | cyclooxygenase |
| CTGF | connective tissue growth factor |
| CyA | cyclosporine A |
| DM | diabetes mellitus |
| DR | diabetic retinopathy |
| EC | endothelial cells |
| Egr-1 | early growth response protein 1 |
| eNOS | endothelial nitric oxide synthase |
| EP | ethyl pyruvate |
| ERK | extracellular signal-regulated kinase |
| GA | glycyrrhizin |
| GSH | reduced glutathione |
| HIF-1α | hypoxia induced factor-1α |
| HMGB | high-mobility group box |
| HO-1 | heme oxygenase-1 |
| HRECs | human retinal endothelial cells |
| HRMEC | human retinal microvascular endothelial cells |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFN | interferon |
| IGFBP-3 | insulin-like growth factor-binding protein-3 |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| MAPK | mitogen-activated protein kinase |
| MMP-9 | metalloproteinases-9 |
| NF-κB | nuclear factor-κB |
| NOX | nicotinamide adenine dinucleotide phosphate oxidase |
| NPDR | non proliferative diabetic retinopathy |
| OPN | osteopontin |
| PAI-1 | plasminogen activator inhibitor-1 |
| PARP | poly ADP-ribose polymerase |
| PCE | polygonum cuspidatum |
| PDR | proliferative diabetic retinopathy |
| PEDF | pigment epithelium-derived factor |
| PI3K | phosphatidylinositol 3-kinase |
| PK | protein kinase |
| PLA-2 | phospholipases A2 |
| PVR | proliferative vitreoretinopathy |
| RAGE | receptors for AGEs |
| RGC | retinal ganglion cells |
| ROP | retinopathy of prematurity |
| ROS | reactive oxygen species |
| RPE | retinal pigmented epithelium |
| shRNA | short hairpin RNA |
| siRNA | small interfering RNAs |
| SIRT | sirtuin |
| SOCS3 | suppressor of cytokine signaling 3 |
| SOD | superoxide dismutase |
| STAT-3 | signal transducer and activator of transcription-3 |
| pSTAT-3 | phosphorylated STAT-3 |
| STZ | streptozotocin |
| TGF-β1 | transforming growth factor-β1 |
| TLR | toll like receptor |
| TNF-α | tumor necrosis factor-α |
| TYK2 | tyrosine kinase 2 |
| VAP-1 | vascular adhesion protein-1 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Hendrick, A.M.; Gibson, M.V.; Kulshreshtha, A. Diabetic Retinopathy. Prim. Care 2015, 42, 451–464. [Google Scholar] [CrossRef]
- Robles-Rivera, R.R.; Castellanos-González, J.A.; Olvera-Montaño, C.; Flores-Martin, R.A.; López-Contreras, A.K.; Arevalo-Simental, D.E.; Cardona-Muñoz, E.G.; Roman-Pintos, L.M.; Rodríguez-Carrizalez, A.D. Adjuvant Therapies in Diabetic Retinopathy as an Early Approach to Delay Its Progression: The Importance of Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2020, 2020, 3096470. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [Green Version]
- Bandello, F.; Lattanzio, R.; Zucchiatti, I.; Del Turco, C. Pathophysiology and treatment of diabetic retinopathy. Acta Diabetol. 2013, 50, 1–20. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019. [Google Scholar] [CrossRef]
- Stitt, A.W. AGEs and Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4867–4874. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Abbas, S.N. Diabetes-induced mitochondrial dysfunction in the retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5327–5334. [Google Scholar] [CrossRef] [Green Version]
- Chalupsky, K.; Cai, H. Endothelial dihydrofolate reductase: Critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2005, 102, 9056–9061. [Google Scholar] [CrossRef] [Green Version]
- Geraldes, P.; Hiraoka-Yamamoto, J.; Matsumoto, M.; Clermont, A.; Leitges, M.; Marette, A.; Aiello, L.P.; Kern, T.S.; King, G.L. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009, 15, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, H.P. Diabetic retinopathy: Hyperglycemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Taguchi, T.; Matsumura, T.; Pestell, R.; Edelstein, D.; Giardino, I.; Suske, G.; Rabbani, N.; Thornalley, P.J.; Sarthy, V.P.; et al. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J. Biol. Chem. 2007, 282, 31038–31045. [Google Scholar] [CrossRef] [Green Version]
- Rajamani, U.; Jialal, I. Hyperglycemia induces Toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells: Implications for diabetic retinopathy. J. Diabetes Res. 2014, 2014, 790902. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, K.; Yu, S.J.; Li, Q.; Li, N.; Lin, P.Y.; Li, M.M.; Guo, J.Y. Association of the TLR4 signaling pathway in the retina of streptozotocin-induced diabetic rats. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 389–398. [Google Scholar] [CrossRef]
- PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: Initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes 2005, 54, 2188–2197. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. (Lond.) 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, G.H.; Sanders, C.; Johns, E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 1973, 38, 14–19. [Google Scholar] [CrossRef]
- Tripathi, A.; Shrinet, K.; Kumar, A. HMGB1 protein as a novel target for cancer. Toxicol. Rep. 2019, 6, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Aspects Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Stumbo, A.C.; Cortez, E.; Rodrigues, C.A.; Henriques, M.D.; Porto, L.C.; Barbosa, H.S.; Carvalho, L. Mitochondrial localization of non-histone protein HMGB1 during human endothelial cell-Toxoplasma gondii infection. Cell Biol. Int. 2008, 32, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Kang, R.; Zeh, H.J., 3rd; Lotze, M.T. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef] [Green Version]
- Venereau, E.; Casalgrandi, M.; Schiraldi, M.; Antoine, D.J.; Cattaneo, A.; De Marchis, F.; Liu, J.; Antonelli, A.; Preti, A.; Raeli, L.; et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012, 209, 1519–1528. [Google Scholar] [CrossRef] [Green Version]
- Kazama, H.; Ricci, J.E.; Herndon, J.M.; Hoppe, G.; Green, D.R.; Ferguson, T.A. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008, 29, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandke, P.; Vasquez, K.M. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: Implications in DNA repair and immune responses. DNA Repair (Amst.) 2019, 83, 102701. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J., 3rd; et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciucci, A.; Gabriele, I.; Percario, Z.A.; Affabris, E.; Colizzi, V.; Mancino, G. HMGB1 and cord blood: Its role as immuno-adjuvant factor in innate immunity. PLoS ONE 2011, 6, e23766. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wang, J.; Zhu, F.; Li, R.; Liu, B.; Xu, W.; He, G.; Cao, H.; Wang, Y.; Yang, J. HMGB1 regulates T helper 2 and T helper17 cell differentiation both directly and indirectly in asthmatic mice. Mol. Immunol. 2018, 97, 45–55. [Google Scholar] [CrossRef]
- Lee, G.; Espirito Santo, A.I.; Zwingenberger, S.; Cai, L.; Vogl, T.; Feldmann, M.; Horwood, N.J.; Chan, J.K.; Nanchahal, J. Fully reduced HMGB1 accelerates the regeneration of multiple tissues by transitioning stem cells to GAlert. Proc. Natl. Acad. Sci. USA 2018, 115, E4463–E4472. [Google Scholar] [CrossRef] [Green Version]
- Andersson, U.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 2002, 72, 1084–1091. [Google Scholar]
- Park, J.S.; Arcaroli, J.; Yum, H.K.; Yang, H.; Wang, H.; Yang, K.Y.; Choe, K.H.; Strassheim, D.; Pitts, T.M.; Tracey, K.J.; et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell. Physiol. 2003, 284, C870–C879. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; You, H.; Li, X.M.; Liu, T.H.; Wang, P.; Wang, B.E. HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pac. J. Cancer Prev. 2012, 13, 1365–1370. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Mi, Y.; Yang, H.; Hu, A.; Zhang, Q.; Shang, C. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-κB pathway. Mol. Cell. Biochem. 2013, 380, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, E.; Fasth, A.E.; Palmblad, K.; Harris, H.E.; Andersson, U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology 2009, 214, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Degryse, B.; de Virgilio, M. The nuclear protein HMGB1, a new kind of chemokine? FEBS Lett. 2003, 553, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Schlueter, C.; Weber, H.; Meyer, B.; Rogalla, P.; Röser, K.; Hauke, S.; Bullerdiek, J. Angiogenetic signaling through hypoxia: HMGB1: An angiogenetic switch molecule. Am. J. Pathol. 2005, 166, 1259–1263. [Google Scholar] [CrossRef]
- Yang, S.; Xu, L.; Yang, T.; Wang, F. High-mobility group box-1 and its role in angiogenesis. J. Leukoc. Biol. 2014, 95, 563–574. [Google Scholar] [CrossRef]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Ju, Z.; Ragab, A.A.; Lundbäck, P.; Long, W.; Valdes-Ferrer, S.I.; He, M.; Pribis, J.P.; Li, J.; et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J. Exp. Med. 2015, 212, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef] [Green Version]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cellstriggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Toure, F.; Zahm, J.M.; Garnotel, R.; Lambert, E.; Bonnet, N.; Schmidt, A.M.; Vitry, F.; Chanard, J.; Gillery, P.; Rieu, P. Receptor for advanced glycationend-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem. J. 2008, 416, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, H.; Chen, F.; Wang, Y.; Ma, J.; Wang, G. The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp. Ther. Med. 2019, 17, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Massey, N.; Puttachary, S.; Bhat, S.M.; Kanthasamy, A.G.; Charavaryamath, C. HMGB1-RAGE Signaling Plays a Role in Organic Dust-Induced Microglial Activation and Neuroinflammation. Toxicol. Sci. 2019, 169, 579–592. [Google Scholar] [CrossRef]
- Wolfson, R.K.; Chiang, E.T.; Garcia, J.G. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc. Res. 2011, 81, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Bangert, A.; Andrassy, M.; Müller, A.M.; Bockstahler, M.; Fischer, A.; Volz, C.H.; Leib, C.; Göser, S.; Korkmaz-Icöz, S.; Zittrich, S.; et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc. Natl. Acad. Sci. USA 2016, 113, E155–E164. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007, 81, 741–747. [Google Scholar] [CrossRef]
- Mitola, S.; Belleri, M.; Urbinati, C.; Coltrini, D.; Sparatore, B.; Pedrazzi, M.; Melloni, E.; Presta, M. Cutting edge: Extracellular high mobility group box-1 protein is a proangiogenic cytokine. J. Immunol. 2006, 176, 12–15. [Google Scholar] [CrossRef]
- van Beijnum, J.R.; Nowak-Sliwinska, P.; van den Boezem, E.; Hautvast, P.; Buurman, W.A.; Griffioen, A.W. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene 2013, 32, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Z.; Zheng, J.J.; Bai, Y.H.; Xia, P.; Zhang, H.C.; Guo, Y. HMGB1/RAGE axis mediates the apoptosis, invasion, autophagy, and angiogenesis of the renal cell carcinoma. OncoTargets Ther. 2018, 11, 4501–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Yang, X.P.; Fang, D.; Ren, X.; Zhou, H.; Fang, J.; Liu, X.; Zhou, S.; Wen, F.; Yao, X.; et al. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1024–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Jiang, P.; Yang, R.; Liu, D.F. Functional role of SIRT1-induced HMGB1 expression and acetylation in migration, invasion and angiogenesis of ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.W.; Kim, H.Y.; Lee, W.S.; Hong, K.W.; Kim, C.D. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. Eur. J. Immunol. 2015, 45, 1216–1227. [Google Scholar] [CrossRef]
- Mohammad, G.; Siddiquei, M.M.; Othman, A.; Al-Shabrawey, M.; Abu El-Asrar, A.M. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp. Eye Res. 2013, 107, 101–109. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Sohn, E.; Kim, J.S. Cytoplasmic translocation of high-mobility group box-1 protein is induced by diabetes and high glucose in retinal pericytes. Mol. Med. Rep. 2016, 14, 3655–3661. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Jomar, D.; Siddiquei, M.M.; Alam, K.; Abu El-Asrar, A.M. High-Mobility Group Box-1 Protein Mediates the Regulation of Signal Transducer and Activator of Transcription-3 in the Diabetic Retina and in Human Retinal Müller Cells. Ophthalmic Res. 2017, 57, 150–160. [Google Scholar] [CrossRef]
- Mohammad, G.; Alam, K.; Nawaz, M.I.; Siddiquei, M.M.; Mousa, A.; Abu El-Asrar, A.M. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J. Physiol. Biochem. 2015, 71, 359–372. [Google Scholar] [CrossRef]
- Ran, R.J.; Zheng, X.Y.; Du, L.P.; Zhang, X.D.; Chen, X.L.; Zhu, S.Y. Upregulated inflammatory associated factors and blood-retinal barrier changes in the retina of type 2 diabetes mellitus model. Int. J. Ophthalmol. 2016, 9, 1591–1597. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, J.; Zhang, H. Role of Sirtuin 1 in the pathogenesis of ocular disease (Review). Int. J. Mol. Med. 2018, 42, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Abdelaziz, G.M.; Siddiquei, M.M.; Ahmad, A.; De Hertogh, G.; Abu El-Asrar, A.M. Cross-Talk between Sirtuin 1 and the Proinflammatory Mediator High-Mobility Group Box-1 in the Regulation of Blood-Retinal Barrier Breakdown in Diabetic Retinopathy. Curr. Eye Res. 2019, 44, 1133–1143. [Google Scholar] [CrossRef]
- Liu, L.; Patel, P.; Steinle, J.J. PKA regulates HMGB1 through activation of IGFBP-3 and SIRT1 in human retinal endothelial cells cultured in high glucose. Inflamm. Res. 2018, 67, 1013–1019. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, X.D.; Li, Y.Y.; Chen, X.M.; Tang, D.R.; Ran, R.J. Involvement of HMGB1 mediated signalling pathway in diabetic retinopathy: Evidence from type 2 diabetic rats and ARPE-19 cells under diabetic condition. Br. J. Ophthalmol. 2013, 97, 1598–1603. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, X. Expression of High-Mobility Group Box 1 Protein (HMGB1) and Toll-Like Receptor 9 (TLR9) in Retinas of Diabetic Rats. Med. Sci. Monit. 2017, 23, 3115–3122. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhang, J.; Yu, J. HMGB-1 as a Potential Target for the Treatment of Diabetic Retinopathy. Med. Sci. Monit. 2015, 21, 3062–3067. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, L.; Lv, J.; Huang, X.; Yi, J.; Pei, C.; Shao, Y. The role of high mobility group box 1 (HMGB-1) in the diabetic retinopathy inflammation and apoptosis. Int. J. Clin. Exp. Pathol. 2015, 8, 6807–6813. [Google Scholar]
- Gong, Y.; Jin, X.; Wang, Q.S.; Wei, S.H.; Hou, B.K.; Li, H.Y.; Zhang, M.N.; Li, Z.H. The involvement of high mobility group 1 cytokine and phospholipases A2 in diabetic retinopathy. Lipids Health Dis. 2014, 13, 156. [Google Scholar] [CrossRef] [Green Version]
- van Beijnum, J.R.; Buurman, W.A.; Griffioen, A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008, 11, 91–99. [Google Scholar] [CrossRef]
- Santos, A.R.; Dvoriantchikova, G.; Li, Y.; Mohammad, G.; Abu El-Asrar, A.M.; Wen, R.; Ivanov, D. Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PLoS ONE 2014, 9, e87574. [Google Scholar] [CrossRef]
- Lee, J.J.; Hsiao, C.C.; Yang, I.H.; Chou, M.H.; Wu, C.L.; Wei, Y.C.; Chen, C.H.; Chuang, J.H. High-mobility group box 1 protein is implicated in advanced glycation end products-induced vascular endothelial growth factor A production in the rat retinal ganglion cell line RGC-5. Mol. Vis. 2012, 18, 838–850. [Google Scholar]
- Fu, D.; Tian, X. Effect of high mobility group box 1 on the human retinal pigment epithelial cell in high-glucose condition. Int. J. Clin. Exp. Med. 2015, 8, 17796–17803. [Google Scholar]
- Jiang, N.; Chen, X. Protective effect of high mobility group box-1 silence on diabetic retinopathy: An in vivo study. Int. J. Clin. Exp. Pathol. 2017, 10, 8148–8160. [Google Scholar]
- Liang, W.J.; Yang, H.W.; Liu, H.N.; Qian, W.; Chen, X.L. HMGB1 upregulates NF-kB by inhibiting IKB-α and associates with diabetic retinopathy. Life Sci. 2020, 241, 117146. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Alam, K.; Garcia-Ramirez, M.; Ahmad, A.; Siddiquei, M.M.; Mohammad, G.; Mousa, A.; De Hertogh, G.; Opdenakker, G.; Simó, R. Association of HMGB1 with oxidative stress markers and regulators in PDR. Mol. Vis. 2017, 23, 853–871. [Google Scholar]
- El-Asrar, A.M.; Missotten, L.; Geboes, K. Expression of high-mobility groups box-1/receptor for advanced glycation end products/osteopontin/early growth response-1 pathway in proliferative vitreoretinal epiretinal membranes. Mol. Vis. 2011, 17, 508–518. [Google Scholar]
- Abu El-Asrar, A.M.; Imtiaz Nawaz, M.; Kangave, D.; Siddiquei, M.M.; Geboes, K. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediat. Inflamm. 2012, 2012, 493043. [Google Scholar] [CrossRef]
- El-Asrar, A.M.; Nawaz, M.I.; Kangave, D.; Geboes, K.; Ola, M.S.; Ahmad, S.; Al-Shabrawey, M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol. Vis. 2011, 17, 1829–1838. [Google Scholar]
- Shen, Y.; Cao, H.; Chen, F.; Suo, Y.; Wang, N.; Xu, X. A cross-sectional study of vitreous and serum high mobility group box-1 levels in proliferative diabetic retinopathy. Acta Ophthalmol. 2020, 98, e212–e216. [Google Scholar] [CrossRef] [Green Version]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Siddiquei, M.M.; Al-Kharashi, A.S.; Kangave, D.; Mohammad, G. High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediat. Inflamm. 2013, 2013, 863036. [Google Scholar] [CrossRef] [Green Version]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Kangave, D.; Abouammoh, M.; Mohammad, G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediat. Inflamm. 2012, 2012, 697489. [Google Scholar] [CrossRef] [Green Version]
- Abu El-Asrar, A.M.; Siddiquei, M.M.; Nawaz, M.I.; Geboes, K.; Mohammad, G. The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediat. Inflamm. 2014, 2014, 746415. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M. High-Mobility Group Box-1 Modulates the Expression of Inflammatory and Angiogenic Signaling Pathways in Diabetic Retina. Curr. Eye Res. 2015, 40, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, C.W.; Hsieh, M.C.; Wu, H.J.; Wu, W.S.; Wu, W.C.; Kao, Y.H. High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial ARPE-19 cells. Cell Signal. 2017, 40, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H. Molecular biology of secondary metabolism: Case study for Glycyrrhiza plants. In Recent advances in plant biotechnology; Kirakosyan, A., Kaufman, P.B., Eds.; Springer: New York, NY, USA, 2009; pp. 89–103. [Google Scholar]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin Binds to High-Mobility Group Box 1 Protein and Inhibits Its Cytokine Activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, Z.; Aalami, A.; Tohidfar, M.; Sohani, M.M. Metabolic Engineering of Glycyrrhizin Pathway by Over-Expression of Beta-amyrin 11-Oxidase in Transgenic Roots of Glycyrrhiza glabra. Mol. Biotechnol. 2018, 60, 412–419. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Steinle, J.J. Glycyrrhizin Protects the Diabetic Retina against Permeability, Neuronal, and Vascular Damage through Anti-Inflammatory Mechanisms. J. Clin. Med. 2019, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Jiang, Y.; Steinle, J.J. Inhibition of HMGB1 protects the retina from ischemia-reperfusion, as well as reduces insulin resistance proteins. PLoS ONE 2017, 12, e0178236. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Steinle, J.J. Epac1 and Glycyrrhizin Both Inhibit HMGB1 Levels to Reduce Diabetes-Induced Neuronal and Vascular Damage in the Mouse Retina. J. Clin. Med. 2019, 8, 772. [Google Scholar] [CrossRef] [Green Version]
- Fukui, M.; Kitagawa, Y.; Nakamura, N.; Yoshikawa, T. Glycyrrhizin and serum testosterone concentrations in male patients with type 2 diabetes. Diabetes Care 2003, 26, 2962. [Google Scholar] [CrossRef] [Green Version]
- Selvam, C.; Mutisya, D.; Prakash, S.; Ranganna, K.; Thilagavathi, R. Therapeutic potential of chemically modified siRNA: Recent trends. Chem. Biol. Drug Des. 2017, 90, 665–678. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W. RNAi therapeutics: A potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, X. HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo. Drug Des. Dev. Ther. 2017, 11, 783–795. [Google Scholar] [CrossRef] [Green Version]
- Bralley, E.E.; Greenspan, P.; Hargrove, J.L.; Wicker, L.; Hartle, D.K. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J. Inflamm. (Lond.) 2008, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef]
- Han, J.H.; Koh, W.; Lee, H.J.; Lee, H.J.; Lee, E.O.; Lee, S.J.; Khil, J.H.; Kim, J.T.; Jeong, S.J.; Kim, S.H. Analgesic and anti-inflammatory effects of ethyl acetate fraction of Polygonum cuspidatum in experimental animals. Immunopharmacol. Immunotoxicol. 2012, 34, 191–195. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.S.; Lee, Y.M.; Kim, J.S. Extract of polygonum cuspidatum attenuates diabetic retinopathy by inhibiting the high-mobility group box-1 (HMGB1) signaling pathway in streptozotocin-induced diabetic rats. Nutrients 2016, 8, 140. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Wei, W.; Zhu, L.; Liu, J.X. Effects and mechanisms of paeoniflorin, a bioactive glucoside from paeony root, on adjuvant arthritis in rats. Inflamm. Res. 2007, 56, 182–188. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, Y.; Wang, J.; Wei, S.; Wei, Z.; Li, R.; Zhu, Y.; Sun, Z.; Xiao, X. Comparative pharmacokinetic study of paeoniflorin and albiflorin after oral administration of Radix Paeoniae Rubra in normal rats and the acute cholestasis hepatitis rats. Fitoterapia 2012, 83, 415–421. [Google Scholar] [CrossRef]
- Iwahara, N.; Hisahara, S.; Kawamata, J.; Matsumura, A.; Yokokawa, K.; Saito, T.; Fujikura, M.; Manabe, T.; Suzuki, H.; Matsushita, T. Role of suppressor of cytokine signaling 3 (SOCS3) in altering activated microglia phenotype in APPswe/PS1dE9 mice. J. Alzheimer’s Dis. JAD 2016, 55, 1235–1247. [Google Scholar] [CrossRef]
- Zhu, S.H.; Liu, B.Q.; Hao, M.J.; Fan, Y.X.; Qian, C.; Teng, P.; Zhou, X.W.; Hu, L.; Liu, W.T.; Yuan, Z.L.; et al. Paeoniflorin Suppressed High Glucose-Induced Retinal Microglia MMP-9 Expression and Inflammatory Response via Inhibition of TLR4/NF-κB Pathway Through Upregulation of SOCS3 in Diabetic Retinopathy. Inflammation 2017, 40, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ward, P.A.; Wang, X.; Gao, H. Myeloid depletion of SOCS3 enhances LPS-induced acute lung injury through CCAAT/enhancer binding protein δ pathway. FASEB J. 2013, 27, 2967–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the effectiveness of willow bark for musculoskeletal pain. Phytother. Res. 2009, 23, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, K.H.; Choi, J.S.; Kim, J.E.; Park, C.; Jeong, J.W. Salicin, an extract from white willow bark, inhibits angiogenesis by blocking the ROS-ERK pathways. Phytother. Res. 2014, 28, 1246–1251. [Google Scholar] [CrossRef]
- Ishikado, A.; Sono, Y.; Matsumoto, M.; Robida-Stubbs, S.; Okuno, A.; Goto, M.; King, G.L.; Keith Blackwell, T.; Makino, T. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic. Biol. Med. 2013, 65, 1506–1515. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Tian, X.; Wang, X.; Feng, H. Vascular protection of salicin on IL-1β-induced endothelial inflammatory response and damages in retinal endothelial cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1995–2002. [Google Scholar] [CrossRef] [Green Version]
- Fink, M.P. Ethyl pyruvate: A novel anti-inflammatory agent. J. Intern. Med. 2007, 261, 349–362. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, J.; Jo, K.; Shin, S.D.; Kim, C.S.; Sohn, E.J.; Kim, S.G.; Kim, J.S. Ethyl pyruvate inhibits retinal pathogenic neovascularization by downregulating HMGB1 expression. J. Diabetes Res. 2013, 2013, 245271. [Google Scholar] [CrossRef]
- Maurer, M.; Bader, M.; Bas, M.; Bossi, F.; Cicardi, M.; Cugno, M.; Howarth, P.; Kaplan, A.; Kojda, G.; Leeb-Lundberg, F.; et al. New topics in bradykinin research. Allergy 2011, 66, 1397–1406. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.Y.; Wang, J.; Zhu, Y.G. Bradykinin alleviates DR retinal endothelial injury by regulating HMGB-1/NF-κB pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5535–5541. [Google Scholar] [CrossRef]
- Zhao, L.; Patel, S.H.; Pei, J.; Zhang, K. Antagonizing Wnt pathway in diabetic retinopathy. Diabetes 2013, 62, 3993–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, J.; Bledsoe, G.; Chao, L. Protective Role of Kallistatin in Vascular and Organ Injury. Hypertension 2016, 68, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.X.; King, L.P.; Yang, Z.; Crouch, R.K.; Chao, L.; Chao, J. Kallistatin in human ocular tissues: Reduced levels in vitreous fluids from patients with diabetic retinopathy. Curr. Eye Res. 1996, 15, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, G.; Kang, L.; Wu, J.; Chen, H.; Liu, G.; Zhu, R.; Guan, H.; Lu, P. The Suppression of Kallistatin on High-Glucose-Induced Proliferation of Retinal Endothelial Cells in Diabetic Retinopathy. Ophthalmic Res. 2017, 57, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guy, K.; Pagadala, J.; Jiang, Y.; Walker, R.J.; Liu, L.; Soderland, C.; Kern, T.S.; Ferry, R., Jr.; He, H.; et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3004–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Pagadala, J.; Miller, D.; Steinle, J.J. Reduced insulin receptor signaling in retinal Müller cells cultured in high glucose. Mol. Vis. 2013, 19, 804–811. [Google Scholar]
- Berger, E.A.; Carion, T.W.; Jiang, Y.; Liu, L.; Chahine, A.; Walker, R.J.; Steinle, J.J. β-Adrenergic receptor agonist, compound 49b, inhibits TLR4 signaling pathway in diabetic retina. Immunol. Cell Biol. 2016, 94, 656–661. [Google Scholar] [CrossRef]
- Nebbioso, M.; Alisi, L.; Giovannetti, F.; Armentano, M.; Lambiase, A. Eye drop emulsion containing 0.1% cyclosporin (1 mg/mL) for the treatment of severe vernal keratoconjunctivitis: An evidence-based review and place in therapy. Clin. Ophthalmol. 2019, 13, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chen, F.; Zhang, X. Cyclosporine-a attenuates retinal inflammation by inhibiting HMGB-1 formation in rats with type 2 diabetes mellitus. BMC Pharmacol. Toxicol. 2020, 21, 9. [Google Scholar] [CrossRef] [Green Version]
- Carmo, A.; Cunha-Vaz, J.G.; Carvalho, A.P.; Lopes, M.C. Effect of cyclosporin-A on the blood-retinal barrier permeability in streptozotocin-induced diabetes. Mediat. Inflamm. 2000, 9, 243–248. [Google Scholar] [CrossRef]





| Drug | Target Test | Diabetic Inducement | Mechanism of Action | Results | Reference |
|---|---|---|---|---|---|
| Glycyrrhizin | HRECs | High glucose concentrations | Inhibition of TLR-4 and TNF-α; cleavage of caspase 3 through inactivation of HMGB1 | Increased insulin receptor signal transduction | [99,100] |
| Mice | Ischemia/reperfusion damage | Block of the loss of retinal thickness | Protects GCL and retinal capillaries | [99,100] | |
| Mice | STZ | Upregulation of SIRT1, inhibition of inflammatory factors. Attenuates BDNF downregulation, reduces ROS, ICAM-1, NF-κB, and HIF-1α | Reduced vascular permeability, increased retinal thickness. Protection from diabetes-induced retinal damages and inflammation | [101] [67,68,85,90] [93] | |
| Retinal Muller Cells | High glucose concentrations | Attenuates p-STAT3 expression | Inhibition of VEGF expression | [69] | |
| Small interfering RNAs | Mice | STZ | Intravitreal injection of HMGB1 siRNA | Protected morphological changes, and improved the function of the retina | [105] |
| Retinal ganglion cells | High glucose concentrations | Transfection with HMGB1 siRNA reduced the expression of TLR-4 and NF-κB | Increased cell survival rate | [77] | |
| HRECs | High glucose concentrations | Transfection with HMGB1 siRNA reduced the expression of caspase 3 | Inhibition the early stage of apoptosis | [105] | |
| Short hairpin RNAs | Rat retinas | High glucose concentrations | Transfection with HMGB1 shRNA reduced the expression TNF-α and NF-κB | Increased cell survival rate and vascular permeability | [83] |
| Polygonum cuspidatum | Mice | STZ | Reduced RAGE and NF-κB expression | Reduced vascular permeability | [109] |
| Paeoniflorin | Mice | STZ | Inhibition of MMP-9 and IL-1β | Alleviated microglial activation | [113] |
| BV2 modified microglial cells | High glucose concentrations | Inhibition of NF-κB expression and SOCS3 | Reduced MMP-9 and TLR-4 concentrations | [113] | |
| Salicin | HRECs | Incubated with IL-1β (inflammatory response) | Suppression of NF-κB pathway and the release of MMP | Inhibition of IL-1β mediated inflammatory pathways | [118] |
| Ethyl pyruvate | Mice | Induction of ROP through exposition to hyperoxia | Reduction of ROS, NF-κB, IL-6, VEGF and TNF-α | Reduction of neoangiogenesis and areas of ischemic retina | [120] |
| Bradykinin | HRECs | High glucose concentrations | Suppression of NF-κB, caspase 3, VEGF, TNF-α, IL-1β. Increase in SOD activity | Promotion of retinal cells survival/ inhibition of apoptosis. Reduction of vascular permeability | [122] |
| Kallistatin | HRECs | High glucose concentrations | Suppression of VEGF expression | Reduction of neoangiogenesis | [126] |
| Compound 49b | HRECs and rat retinal Muller cells | High glucose concentrations | Increase of IGFBP-3 levels and inhibition of TLR-4 pathway | Prevention of cellular apoptosis | [127,129] |
| Mice | SZT | Increase of IGFBP-3 levels | Prevention of the decrease in retinal thickness and loss of cells in GCL | [127] | |
| Cyclosporine A | Mice | STZ | Reduction of TNF-α and IL-1β | Amelioration of retinal thickness, regression of retinal edema | [131] |
| Mice | STZ | Reduction of iNOS, IL-1β and COX-2 | Reduction of BRB permeability | [132] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebbioso, M.; Lambiase, A.; Armentano, M.; Tucciarone, G.; Bonfiglio, V.; Plateroti, R.; Alisi, L. The Complex Relationship between Diabetic Retinopathy and High-Mobility Group Box: A Review of Molecular Pathways and Therapeutic Strategies. Antioxidants 2020, 9, 666. https://doi.org/10.3390/antiox9080666
Nebbioso M, Lambiase A, Armentano M, Tucciarone G, Bonfiglio V, Plateroti R, Alisi L. The Complex Relationship between Diabetic Retinopathy and High-Mobility Group Box: A Review of Molecular Pathways and Therapeutic Strategies. Antioxidants. 2020; 9(8):666. https://doi.org/10.3390/antiox9080666
Chicago/Turabian StyleNebbioso, Marcella, Alessandro Lambiase, Marta Armentano, Giosuè Tucciarone, Vincenza Bonfiglio, Rocco Plateroti, and Ludovico Alisi. 2020. "The Complex Relationship between Diabetic Retinopathy and High-Mobility Group Box: A Review of Molecular Pathways and Therapeutic Strategies" Antioxidants 9, no. 8: 666. https://doi.org/10.3390/antiox9080666