Abstract
Purpose
More than 50 different monogenic disorders causing inflammatory bowel disease (IBD) have been identified. Our goal was to characterize the clinical phenotype, genetic workup, and immunologic alterations in an Ashkenazi Jewish patient that presented during infancy with ulcerative colitis and unique clinical manifestations.
Methods
Immune workup and whole-exome sequencing were performed, along with Sanger sequencing for confirmation. Next-generation sequencing of the TCRB and IgH was conducted for immune repertoire analysis. Telomere length was evaluated by in-gel hybridization assay. Mass cytometry was performed on patient’s peripheral blood mononuclear cells, and compared with control subjects and patients with UC.
Results
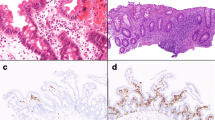
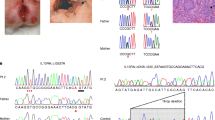
The patient presented in infancy with failure to thrive and dysmorphic features, consistent with a diagnosis of dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Severe ulcerative colitis manifested in the first year of life and proceeded to the development of a primary immunodeficiency, presenting as Pneumocystis jiroveci pneumonia and hypogammaglobulinemia. Genetic studies identified a deleterious homozygous C.3791G>A missense mutation in the helicase regulator of telomere elongation 1 (RTEL1), leading to short telomeres in the index patient. Immune repertoire studies showed polyclonal T and B cell receptor distribution, while mass cytometry analysis demonstrated marked immunological alterations, including a predominance of naïve T cells, paucity of B cells, and a decrease in various innate immune subsets.
Conclusions
RTEL1 mutations are associated with significant alterations in immune landscape and can manifest with infantile-onset IBD. A high index of suspicion is required in Ashkenazi Jewish families where the carriage rate of the C.3791G>A variant is high.




Similar content being viewed by others
References
Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578:527–39. https://doi.org/10.1038/s41586-020-2025-2.
Uhlig, H. H. et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 147, 990–1007. e1003 (2014).
Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015;1:462–76. https://doi.org/10.1016/j.jcmgh.2015.06.010.
Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2013;6:327–37. https://doi.org/10.1586/ehm.13.23.
Roake CM, Artandi SE. Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol. 2020;21:384–97. https://doi.org/10.1038/s41580-020-0234-z.
Tangye SG, al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40:24–64. https://doi.org/10.1007/s10875-019-00737-x.
Savage, S. A. Beginning at the ends: telomeres and human disease. F1000Res 7, doi:https://doi.org/10.12688/f1000research.14068.1 (2018).
Fernandez Garcia MS, Teruya-Feldstein J. The diagnosis and treatment of dyskeratosis congenita: a review. J Blood Med. 2014;5:157–67. https://doi.org/10.2147/JBM.S47437.
Glousker G, Touzot F, Revy P, Tzfati Y, Savage SA. Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder. Br J Haematol. 2015;170:457–71. https://doi.org/10.1111/bjh.13442.
Werner L, Lee YN, Rechavi E, Lev A, Yerushalmi B, Ling G, et al. Alterations in T and B cell receptor repertoires patterns in patients with IL10 signaling defects and history of infantile-onset IBD. Front Immunol. 2020;11:109. https://doi.org/10.3389/fimmu.2020.00109.
Lamm N, Ordan E, Shponkin R, Richler C, Aker M, Tzfati Y. Diminished telomeric 3′ overhangs are associated with telomere dysfunction in Hoyeraal-Hreidarsson syndrome. PLoS One. 2009;4:e5666. https://doi.org/10.1371/journal.pone.0005666.
Sagie S, Ellran E, Katzir H, Shaked R, Yehezkel S, Laevsky I, et al. Induced pluripotent stem cells as a model for telomeric abnormalities in ICF type I syndrome. Hum Mol Genet. 2014;23:3629–40. https://doi.org/10.1093/hmg/ddu071.
Konnikova L, Boschetti G, Rahman A, Mitsialis V, Lord J, Richmond C, et al. High-dimensional immune phenotyping and transcriptional analyses reveal robust recovery of viable human immune and epithelial cells from frozen gastrointestinal tissue. Mucosal Immunol. 2018;11:1684–93. https://doi.org/10.1038/s41385-018-0047-y.
Ballew BJ, Joseph V, de S, Sarek G, Vannier JB, Stracker T, et al. A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome. PLoS Genet. 2013;9:e1003695. https://doi.org/10.1371/journal.pgen.1003695.
Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. Am J Hum Genet. 2013;92:448–53. https://doi.org/10.1016/j.ajhg.2013.02.001.
Deng Z, Glousker G, Molczan A, Fox AJ, Lamm N, Dheekollu J, et al. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 2013;110:E3408–16. https://doi.org/10.1073/pnas.1300600110.
Marsh JCW, Gutierrez-Rodrigues F, Cooper J, Jiang J, Gandhi S, Kajigaya S, et al. Heterozygous RTEL1 variants in bone marrow failure and myeloid neoplasms. Blood Adv. 2018;2:36–48. https://doi.org/10.1182/bloodadvances.2017008110.
Speckmann C, et al. Clinical and molecular heterogeneity of RTEL1 deficiency. Front Immunol. 2017;8:449. https://doi.org/10.3389/fimmu.2017.00449.
Walker JA, et al. Polychromic reporter mice reveal unappreciated innate lymphoid cell progenitor heterogeneity and elusive ILC3 progenitors in bone marrow. Immunity. 2019;51:104–18 e107. https://doi.org/10.1016/j.immuni.2019.05.002.
Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–5. https://doi.org/10.1038/990141.
Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol. 2016;13:696–706. https://doi.org/10.1080/15476286.2015.1094596.
Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, et al. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in dyskeratosis congenita. Hum Genet. 2013;132:473–80. https://doi.org/10.1007/s00439-013-1265-8.
Le Guen T, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013;22:3239–49. https://doi.org/10.1093/hmg/ddt178.
Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell. 2013;12:319–23. https://doi.org/10.1111/acel.12041.
Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet. 2012;44:338–42. https://doi.org/10.1038/ng.1084.
Simon AJ, Lev A, Zhang Y, Weiss B, Rylova A, Eyal E, et al. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J Exp Med. 2016;213:1429–40. https://doi.org/10.1084/jem.20151618.
Bluteau O, Sebert M, Leblanc T, Peffault de Latour R, Quentin S, Lainey E, et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018;131:717–32. https://doi.org/10.1182/blood-2017-09-806489.
Touzot F, Kermasson L, Jullien L, Moshous D, Ménard C, Ikincioğullari A, et al. Extended clinical and genetic spectrum associated with biallelic RTEL1 mutations. Blood Adv. 2016;1:36–46. https://doi.org/10.1182/bloodadvances.2016001313.
Fioredda F, Iacobelli S, Korthof ET, Knol C, van Biezen A, Bresters D, et al. Outcome of haematopoietic stem cell transplantation in dyskeratosis congenita. Br J Haematol. 2018;183:110–8. https://doi.org/10.1111/bjh.15495.
Tamura S, et al. Allogeneic hematopoietic cell transplantation for dyskeratosis congenita: a report of 3 cases. J Pediatr Hematol Oncol. 2017;39:e394–8. https://doi.org/10.1097/MPH.0000000000000844.
Nelson AS, Marsh RA, Myers KC, Davies SM, Jodele S, O’Brien TA, et al. A reduced-intensity conditioning regimen for patients with dyskeratosis congenita undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:884–8. https://doi.org/10.1016/j.bbmt.2016.01.026.
Sorge C, Pereboeva L, Westin E, Harris WT, Kelly DR, Goldman F. Pulmonary complications post hematopoietic stem cell transplant in dyskeratosis congenita: analysis of oxidative stress in lung fibroblasts. Bone Marrow Transplant. 2017;52:765–8. https://doi.org/10.1038/bmt.2016.353.
Chen, R. L., Lin, K. K. & Chen, L. Y. Complications for a Hoyeraal-Hreidarsson syndrome patient with a germline DKC1 A353V variant undergoing unrelated peripheral blood stem cell transplantation. Int J Mol Sci 20:3261, doi:https://doi.org/10.3390/ijms20133261 (2019).
Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922–31. https://doi.org/10.1056/NEJMoa1515319.
Catala A, et al. Androgen therapy in inherited bone marrow failure syndromes: analysis from the Canadian Inherited Marrow Failure Registry. Br J Haematol. 2020;189:976–81. https://doi.org/10.1111/bjh.16445.
Khincha PP, Bertuch AA, Gadalla SM, Giri N, Alter BP, Savage SA. Similar telomere attrition rates in androgen-treated and untreated patients with dyskeratosis congenita. Blood Adv. 2018;2:1243–9. https://doi.org/10.1182/bloodadvances.2018016964.
Woo DH, Chen Q, Yang TLB, Glineburg MR, Hoge C, Leu NA, et al. Enhancing a Wnt-telomere feedback loop restores intestinal stem cell function in a human organotypic model of dyskeratosis congenita. Cell Stem Cell. 2016;19:397–405. https://doi.org/10.1016/j.stem.2016.05.024.
Gu BW, Apicella M, Mills J, Fan JM, Reeves DA, French D, et al. Impaired telomere maintenance and decreased canonical WNT signaling but normal ribosome biogenesis in induced pluripotent stem cells from X-linked dyskeratosis congenita patients. PLoS One. 2015;10:e0127414. https://doi.org/10.1371/journal.pone.0127414.
Raup-Konsavage WM, Cooper TK, Yochum GS. A role for MYC in lithium-stimulated repair of the colonic epithelium after DSS-induced damage in mice. Dig Dis Sci. 2016;61:410–22. https://doi.org/10.1007/s10620-015-3852-0.
Daneshmand A, Mohammadi H, Rahimian R, Habibollahi P, Fakhfouri G, Talab SS, et al. Chronic lithium administration ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats; potential role for adenosine triphosphate sensitive potassium channels. J Gastroenterol Hepatol. 2011;26:1174–81. https://doi.org/10.1111/j.1440-1746.2011.06719.x.
Fedick AM, Shi L, Jalas C, Treff NR, Ekstein J, Kornreich R, et al. Carrier screening of RTEL1 mutations in the Ashkenazi Jewish population. Clin Genet. 2015;88:177–81. https://doi.org/10.1111/cge.12459.
Acknowledgments
We would like to thank the patients and their families for participating in this study. We appreciate the support by the German Academic Exchange Service DAAD (CK, SS, and RS) as well as the Care-for-Rare Foundation.
Authorship Contribution
AZ, TS, BW, RS, and DSS contributed to sample acquisition. AZ, LW, LK, AA, TJ, MH, VM, SW, DK CK, SBS, YT, RS, and DSS contributed to data analysis. DSS designed the study, coordinated research studies, and wrote the manuscript.
Funding
DSS, SBS, and CK are supported by the Leona M. and Harry B. Helmsley Charitable Trust. SBS is also supported by the Wolpow Chair in IBD Research and Treatment and the Translational Research Program (Boston Children’s Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local IRB committee at Sheba Medical Center. Informed written consent was obtained from the parents.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Ziv, A., Werner, L., Konnikova, L. et al. An RTEL1 Mutation Links to Infantile-Onset Ulcerative Colitis and Severe Immunodeficiency. J Clin Immunol 40, 1010–1019 (2020). https://doi.org/10.1007/s10875-020-00829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-020-00829-z