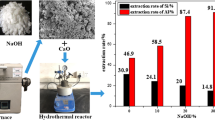
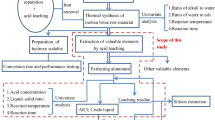
Abstract
Coal fly ash is a lightweight incombustible particulate generated during coal combustion. Alumina content in the fly ash is present in the mullite phase, and its high stability calls for alternative processing for conversion to reactive phase before leaching. Mechanical activation with sodium hydroxide was found unsatisfactory for the breakdown of mullite structure, whereas the mullite phase dissociated to sodium silicate and sodium aluminosilicate during the sodium hydroxide thermal treatment. Below 400 °C, hydroxycancrinite is formed, which leads to alumina loss, whereas above 600 °C, it dissociates to sodium aluminate and sodium silicate, facilitating the alumina extraction. Aluminum hydroxide was recovered by acid leaching followed by precipitation and calcination at 1000 °C to obtain gamma-alumina. The aluminum dissolution increased by incorporating thermal treatment from 5 to 80% in muffle and 89.4% in microwave routes. The acid–base precipitation process resulted in the recovery of ~ 98% dissolved aluminum values in the boehmite phase, leaving the solution with less than 50 ppm Al content suitable for leaching application. Microwave processing was found to be a promising and alternative economical route for the alumina extraction from fly ash.
Graphical Abstract











Similar content being viewed by others
References
Ding J, Ma S, Shen S, Xie Z, Zheng S, Zhang Y (2017) Research and industrialization progress of recovering alumina from fly ash: a concise review. Waste Manag 60:375–387. https://doi.org/10.1016/j.wasman.2016.06.009
Yao Z, Zia MS, Sarker PK, Chen T (2014) A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 120:74–85. https://doi.org/10.1016/j.fuel.2013.12.003
Central Electricity Authority, New Delhi (2018), Report on fly ash generation at coal/lignite based thermal power stations and its utilization in the country report 2018–19. https://www.cea.nic.in/reports/others/thermal/tcd/flyash_201718.pdf. Accessed 01.12.2018
Sibanda V, Ndlovu S, Dombo G et al (2016) Towards the utilization of fly ash as a feedstock for smelter grade alumina production: a review of the developments. J Sustain Metall 2:167–184. https://doi.org/10.1007/s40831-016-0048-6
Sun Y, Liang Z, Sun F, He S, Wang B, Wang Y (2017) Recovery of alumina from coal fly ash by CaCl2 calcination followed by H2SO4 leaching. J Environ Anal Toxicol 7:1–6. https://doi.org/10.4172/2161-0525.1000427
Zhu PW, Dai H, Han L, Xu XL, Cheng LM, Wang QH, Shi ZL (2015) Aluminum extraction from coal ash by a two-step acid leaching method. J Zhejiang Univ Sci A 16(2):161–169
Li H, Hui J, Wang C, Bao W, Sun Z (2014) Extraction of alumina from coal fly ash by mixed-alkaline hydrothermal method. Hydrometallurgy 147–148:183–187. https://doi.org/10.1016/j.hydromet.2014.05.012
Wang L, Zhang TA, Lv GZ, Dou ZH, Zhang WG, Niu LP, Zhang ZM (2019) Kinetics of magnesium and calcium extraction from fly ash by carbochlorination. JOM 71:2798–2805. https://doi.org/10.1007/s11837-019-03474-z
Li S, Qin S, Kang L, Liu J, Wang J, Yanheng L (2017) An efficient approach for lithium and aluminum recovery from coal fly ash by pre-desilication and intensified acid leaching processes. Metal 7:272. https://doi.org/10.3390/met7070272
Tripathy AK, Behera B et al (2019) Sodium fluoride assisted acid leaching of coal fly ash for the extraction of alumina. Miner Eng 131:140–145. https://doi.org/10.1016/j.mineng.2018.10.019
Su SQ, Jing Yang J, Ma HW, Jiang A, Liu YQ, Li G (2011) Preparation of ultrafine aluminum hydroxide from coal fly ash by alkali dissolution process. Integr Ferroelectr 128:155–162. https://doi.org/10.1080/10584587.2011.576626
Xiao J, Li F, Zhong Q, Bao H, Wang B, Huang J, Zhang Y (2015) Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 155:118–124. https://doi.org/10.1016/j.hydromet.2015.04.018
Zhou B, Zhou J, Zhang L, Hu T, Yang L, Lin G (2019) Heating mechanism of high aluminum fly ash activated by Na2CO3 in microwave field. JOM 71(9):2959–2965. https://doi.org/10.1007/s11837-019-03535-3
Tanvar H, Chauhan S, Dhawan N (2018) Extraction of aluminum values from fly ash. Mater Today Proc 5(9):17055–17063. https://doi.org/10.1016/j.matpr.2018.04.112
Matjie RH, Bunt JR, Heerden VHP (2005) Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal. Miner Eng 18(3):299–310. https://doi.org/10.1016/j.mineng.2004.06.013
Valeev D, Kunilova I, Alpatov A, Mikhailova A, Goldberg M, Kondratiev A (2019) Complex utilisation of ekibastuz brown coal fly ash: iron & carbon separation and aluminum extraction. J Clean Prod 218:192–201. https://doi.org/10.1016/j.jclepro.2019.01.342
Agrawal S, Rayapudi V, Dhawan N (2018) Microwave reduction of red mud for recovery of iron values. J Sustain Metall 4(4):427–436. https://doi.org/10.1007/s40831-018-0183-3
Haque KE (1999) Microwave energy for mineral treatment processes—a brief review. Int J Miner Process 57:1–24. https://doi.org/10.1016/S0301-7516(99)00009-5
Querol X, Alastuey A, Lopez-Soler A, Plana F, Andres JM, Juan R, Ruiz CR (1997) A fast method for recycling fly ash: microwave-assisted zeolite synthesis. Environ Sci Technol 31(9):2527–2533. https://doi.org/10.1021/es960937t
Kamarudin RA, Matlob AS, Jubri Z and Ramli Z (2009) Extraction of silica and alumina from coal fly ash for the synthesis of zeolites. In: International conference on energy and environment (ICEE), pp 456–461
Bukhari S, Rohani S (2017) Continuous flow synthesis of zeolite-A from coal fly ash utilizing microwave irradiation with recycled liquid stream. Am J Environ Sci 13(3):233–244. https://doi.org/10.3844/ajessp.2017.233.244
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201(4914):68
Shemi A (2013) Extraction of aluminium from coal fly ash using a two-step acid leach process. Dissertation, University of the Witwatersrand, Johannesburg.
Kumar A, Himanshu T, Dhawan N (2020) Processing of mica for extraction of alumina and potash values. Trans Indian Inst Met 73(1):23–33. https://doi.org/10.1007/s12666-019-01789-8.
Ding J, Shuhua M, Shili Z, Yi Z, Zongli X, Shirley S, Zhongkai L (2016) Study of extracting alumina from high-alumina PC fly ash by a hydro-chemical process. Hydrometallurgy 161:58–64. https://doi.org/10.1016/j.hydromet.2016.01.025
Ding J, Shuhua M, Zongli X, Xiaohui W, Shili Z, Yi Z (2019) Formation mechanism of an undesirable by-product in the mild hydro-chemical process for the extraction of alumina from fly ash and its mitigation. Hydrometallurgy 186:292–300. https://doi.org/10.1016/j.hydromet.2019.04.012
Han YF, Sun TC, Li J, Wang LN, Xue TY, Qi T (2012) Removing of Si in the NaOH molten salt reaction of titanium slag to produce TiO2. Adv Mater Res 418:387–392. https://doi.org/10.4028/www.scientific.net/AMR.418-420.387
Wenk HR, Bulakh A (2003) Minerals—their constitution and origin. Cambridge University Press, Cambridge
Kanwal F, Batool A, Adnan M, Naseem S (2015) The effect of molecular structure, band gap energy and morphology on the dc electrical conductivity of polyaniline/aluminium oxide composites. Mater Res Innov 19(8):354–358. https://doi.org/10.1179/1432891715Z.0000000001688
https://bauxite.world-aluminium.org/refining/energy-efficiency/. Accessed 10 Dec 2019.
He G, Qu W, Zhang L et al (2020) Effects of sodium peroxide additives on dielectric properties and microwave roasting mechanism of zinc sulfide concentrate. JOM 72(5):1920–1926. https://doi.org/10.1007/s11837-020-04050-6
Kingman SW, Rowson NA (1998) Microwave treatment of minerals-a review. Miner Eng 11(11):1081–1087. https://doi.org/10.1016/S0892-6875(98)00094-6
Acknowledgments
The authors acknowledge the financial support received from the Science Engineering Research Board, New Delhi, under extramural funding: EMR/2016/000505.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Agrawal, S. & Dhawan, N. Processing of Coal Fly Ash for the Extraction of Alumina Values. J. Sustain. Metall. 6, 294–306 (2020). https://doi.org/10.1007/s40831-020-00275-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00275-6