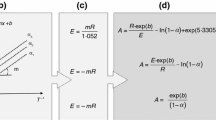
Abstract
The scientific community has shown concern about the suitable application of different methods (model-free/model-fitting) for the determination of kinetic parameters. The application of an unsuitable method may lead to unreliable kinetic parameters. In this study, the performance of five methods for the determination of the kinetic parameters of fodder radish seed cake (FRSC) pyrolysis was evaluated. This is the first detailed study of the pyrolysis kinetics of FRSC. The characterization was performed through thermogravimetric analysis at different heating rates (5, 10, and 25 K/min) and temperatures ranged from 298 to 1073 K. Four model-free isoconversional methods (Friedman, Kissinger, Kissinger–Akahira–Sunose and Flynn–Wall–Ozawa) were used to determine the activation energy. A model-fitting (five pseudo-components model—FPCM) method was used to obtain the kinetic parameters of fodder radish seed cake pyrolysis. A detailed evaluation of the performance of these methods to estimate the kinetic parameters of fodder radish seed cake pyrolysis was performed. The Criado method was applied to verify reaction mechanisms that governed the pyrolysis process. The activation energies (Ea) ranged from 127.70 to 991.53 kJ/mol from the four model-free methods, which presented themselves as unreliable. The FPCM presented a better performance, with higher R2 values. At low conversions, pyrolysis was controlled by diffusion mechanisms, while at higher conversions, a third-order reaction became the governing pyrolysis mechanism.
Graphic abstract








Similar content being viewed by others
Abbreviations
- α:
-
Conversion degree
- β:
-
Heating rate (K/min)
- A :
-
Pre-exponential factor (1/s)
- E a :
-
Activation energy (kJ/mol)
- R:
-
Universal gas constant (J/mol.K)
- R2 :
-
Coefficient of correlation
- c:
-
Mass fraction at a given temperature
- wti :
-
Initial mass (mg)
- wtf :
-
Final mass (mg)
- T:
-
Absolute temperature (K)
- χ:
-
Ea/RT (dimensionless)
- Z(α):
-
Master curve in function of α (Criado method)
- TGA:
-
Thermogravimetric analysis
- DTG:
-
Differential thermogravimetric
References
Ali I, Bahadar A (2017) Red Sea seaweed (Sargassum spp.) pyrolysis and its devolatilization kinetics. Algal Res 21:89–97. https://doi.org/10.1016/j.algal.2016.11.011
ASTM Standard Practice (2013) Standard practice for proximate analysis of coal and coke 1. Annual book of standards. ASTM, West Conshohocken, pp 3174–3175. https://doi.org/10.1520/D3172-13.2
Ávila RNA, Sodré JR (2012) Physical–chemical properties and thermal behavior of fodder radish crude oil and biodiesel. Ind Crops Prod 38:54–57. https://doi.org/10.1016/j.indcrop.2012.01.007
Avrami M (1939) Kinetics of phase change. I general theory. J Chem Phys 7:1103–1112. https://doi.org/10.1063/1.1750380
Bach QV, Chen WH (2017) A comprehensive study on pyrolysis kinetics of microalgal biomass. Energy Convers Manag 131:109–116. https://doi.org/10.1016/j.enconman.2016.10.077
Barros TD, Jardine JG (2016) Nabo Forrageiro [WWW Document]
Bartocci P, Tschentscher R, Stensrød RE, Barbanera M, Fantozzi F (2019) Kinetic analysis of digestate slow pyrolysis with the application of the master-plots method and independent parallel reactions scheme. Molecules 24:1657. https://doi.org/10.3390/molecules24091657
Brachi P, Miccio F, Miccio M, Ruoppolo G (2016) Pseudo-component thermal decomposition kinetics of tomato peels via isoconversional methods. Fuel Process Technol 154:243–250. https://doi.org/10.1016/j.fuproc.2016.09.001
Braga RM, Costa TR, Freitas JCO, Barros JMF, Melo DMA, Melo MAF (2014) Pyrolysis kinetics of elephant grass pretreated biomasses. J Therm Anal Calorim 117:1341–1348. https://doi.org/10.1007/s10973-014-3884-2
Bui HH, Tran KQ, Chen WH (2015) Pyrolysis of microalgae residues—a kinetic study. Bioresour Technol 199:362–366. https://doi.org/10.1016/j.biortech.2015.08.069
Burnham AK (2017) Introduction to chemical kinetics. In: Global chemical kinetics of fossil fuels. Springer, Cham, p 315. https://doi.org/10.1007/978-3-319-49634-4_2
Chammoun N, Geller DP, Das KC (2013) Fuel properties, performance testing and economic feasibility of Raphanus sativus (oilseed radish) biodiesel. Ind Crops Prod 45:155–159. https://doi.org/10.1016/j.indcrop.2012.11.029
Chen J, Fan X, Jiang B, Mu L, Yao P, Yin H, Song X (2015) Pyrolysis of oil-plant wastes in a TGA and a fixed-bed reactor: thermochemical behaviors, kinetics, and products characterization. Bioresour Technol. https://doi.org/10.1016/j.biortech.2015.05.108
Chen C, Miao W, Zhou C, Wu H (2017) Thermogravimetric pyrolysis kinetics of bamboo waste via asymmetric double sigmoidal (Asym2sig) function deconvolution. Bioresour Technol 225:48–57. https://doi.org/10.1016/j.biortech.2016.11.013
Collazzo GC, Broetto CC, Perondi D, Junges J, Dettmer A, Dornelles Filho AA, Foletto EL, Godinho M (2017) A detailed non-isothermal kinetic study of elephant grass pyrolysis from different models. Appl Therm Eng 110:1200–1211. https://doi.org/10.1016/j.applthermaleng.2016.09.012
Correia IMS, Marcelo MJ, De Araújo AS, Sousa EMBD (2012) Thermal stability during pyrolysis of sunflower oil produced in the northeast of Brazil. J Therm Anal Calorim 109:967–974. https://doi.org/10.1007/s10973-011-1773-5
de Carvalho VS, Tannous K (2017) Thermal decomposition kinetics modeling of energy cane Saccharum robustum. Thermochim Acta 657:56–65. https://doi.org/10.1016/j.tca.2017.09.016
De Conto D, Silvestre WP, Baldasso C, Godinho M (2016) Performance of rotary kiln reactor for the elephant grass pyrolysis. Bioresour Technol 218:153–160. https://doi.org/10.1016/j.biortech.2016.06.082
de Matos FC (2012) Estudo da decomposição térmica de Ácidos Graxos através da Calorimetria Exploratória Diferencial. Dissertação 86
de Souza ADV, Fávaro SP, Ítavo LCV, Roscoe R (2009) Caracterização química de sementes e tortas de pinhão-manso, nabo-forrageiro e crambe. Pesqui Agropecuária Bras 44:1328–1335. https://doi.org/10.1590/S0100-204X2009001000017
Diblasi C (2008) Modeling chemical and physical processes of wood and biomass pyrolysis. Prog Energy Combust Sci 34:47–90. https://doi.org/10.1016/j.pecs.2006.12.001
Ferreira SD, Altafini CR, Perondi D, Godinho M (2015) Pyrolysis of medium density fiberboard (MDF) wastes in a screw reactor. Energy Convers Manag 92:223–233. https://doi.org/10.1016/j.enconman.2014.12.032
Ferreira SD, Manera C, Silvestre WPWP, Pauletti GFGF, Altafini CR, Godinho M (2018) Use of biochar produced from elephant grass by pyrolysis in a screw reactor as a soil amendment. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0347-1
Flynn JH, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Polym Lett 4:323–328. https://doi.org/10.1002/pol.1966.110040504
Friedman HL (1964) Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C 6:183–195. https://doi.org/10.1002/polc.5070060121
Gomes V, Júnior F, Calvo A, Edivaldo L (2010) Composição química e digestibilidade do farelo de nabo forrageiro para tilápia do Nilo. Rev Bras Saúde Produção Anim 11:537–546
Ishida Y, Goto K, Yokoi H, Tsuge S, Ohtani H, Sonoda T, Ona T (2007) Direct analysis of phenolic extractives in wood by thermochemolysis–gas chromatography in the presence of tetrabutylammonium hydroxide. J Anal Appl Pyrolysis 78:200–206. https://doi.org/10.1016/j.jaap.2006.06.009
Jandura P, Riedl B, Kokta BV (2000) Thermal degradation behavior of cellulose fibers partially esterified with some long chain organic acids. Polym Degrad Stab 70:387–394. https://doi.org/10.1016/S0141-3910(00)00132-4
Jiang G, Nowakowski DJ, Bridgwater AV (2010) A systematic study of the kinetics of lignin pyrolysis. Acta Thermochim. https://doi.org/10.1016/j.tca.2009.10.003
Junges J, Collazzo GC, Perondi D, Dornelles Filho AA, Ferreira SD, Dettmer A, Osório E, Godinho M (2018a) Critical analysis of non-isothermal kinetics of poultry litter pyrolysis. J Therm Anal Calorim. https://doi.org/10.1007/s10973-018-7710-0
Junges J, Perondi D, Ferreira SD, Dettmer A, Osório E, Godinho M (2018b) Multi-technique characterization of chromated copper arsenate-treated wooden utility poles from the Brazilian electricity network. Eur J Wood Wood Prod. https://doi.org/10.1007/s00107-018-1374-0
Kameno N, Yamada S, Amimoto T, Amimoto K, Ikeda H, Koga N (2016) Thermal degradation of poly(lactic acid) oligomer: reaction mechanism and multistep kinetic behavior. Polym Degrad Stab 134:284–295. https://doi.org/10.1016/j.polymdegradstab.2016.10.018
Kaymak HC (2015) Profile of (n-9) and (n-7) isomers of monounsaturated fatty acids of radish (Raphanus sativus L.) seeds. J Am Oil Chem Soc 92:345–351. https://doi.org/10.1007/s11746-015-2600-0
Kilbourne EM, Rigau-Perez JG, Heath CW Jr, Zack MM, Falk H, Martin-Marcos M, de Carlos A (1983) Clinical epidemiology of toxic-oil syndrome. Manifestations of a new illness. N Engl J Med. https://doi.org/10.1056/NEJM198312083092302
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand (1934) 57:217. https://doi.org/10.6028/jres.057.026
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706. https://doi.org/10.1021/ac60131a045
Lee MK, Tsai WT, Tsai YL, Lin SH (2010) Pyrolysis of napier grass in an induction-heating reactor. J Anal Appl Pyrolysis 88:110–116. https://doi.org/10.1016/j.jaap.2010.03.003
Li B, Lv W, Zhang Q, Wang T, Ma L (2014) Pyrolysis and catalytic pyrolysis of industrial lignins by TG-FTIR: kinetics and products. J Anal Appl Pyrolysis 108:295–300. https://doi.org/10.1016/j.jaap.2014.04.002
Li H, Niu S, Lu C, Wang Y (2015) Comprehensive investigation of the thermal degradation characteristics of biodiesel and its feedstock oil through TGA-FTIR. Energy Fuels 29:5145–5153. https://doi.org/10.1021/acs.energyfuels.5b01054
Li H, Niu S, Lu C (2017a) Thermal characteristics and kinetic calculation of castor oil pyrolysis. Procedia Eng 205:3711–3716. https://doi.org/10.1016/j.proeng.2017.10.297
Li X, Mei Q, Dai X, Ding G (2017b) Effect of anaerobic digestion on sequential pyrolysis kinetics of organic solid wastes using thermogravimetric analysis and distributed activation energy model. Bioresour Technol 227:297–307. https://doi.org/10.1016/j.biortech.2016.12.057
Licitra G, Hernandez TM, Van Soest PJ (1996) Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol. https://doi.org/10.1016/0377-8401(95)00837-3
Liu G, Wright MM, Zhao Q, Brown RC, Wang K, Xue Y (2016) Catalytic pyrolysis of amino acids: comparison of aliphatic amino acid and cyclic amino acid. Energy Convers Manag 112:220–225. https://doi.org/10.1016/j.enconman.2016.01.024
Malavolta E, Vitti GC, de Oliveira SA (1997) Avaliação do estado nutricional das plantas: princípios e aplicações, 2nd edn. Piracicaba
Oladokun O, Ahmad A, Abdullah TAT, Nyakuma BB, Bello AAH, Al-Shatri AH (2016) Multicomponent devolatilization kinetics and thermal conversion of Imperata cylindrica. Appl Therm Eng 105:931–940. https://doi.org/10.1016/j.applthermaleng.2016.04.165
Ozawa T (1971) Kinetics of non-isothermal crystallization. Polymer 12:150–158. https://doi.org/10.1016/0032-3861(71)90041-3
Paes J, Neto M, Lima C, Freitas M, Diniz C (2013) Effects of extractives and ash on natural resistance of four woods to xylophogous termites. Cerne 19:399–405
Papari S, Hawboldt K (2015) A review on the pyrolysis of woody biomass to bio-oil: focus on kinetic models. Renew Sustain Energy Rev 52:1580–1595. https://doi.org/10.1016/j.rser.2015.07.191
Puy N, Murillo R, Navarro MV, López JM, Rieradevall J, Fowler G, Aranguren I, García T, Bartrolí J, Mastral AM (2011) Valorisation of forestry waste by pyrolysis in an auger reactor. Waste Manag 31:1339–1349. https://doi.org/10.1016/j.wasman.2011.01.020
Rueda-Ordónez YJ, Tannous K, Olivares-Gómez E (2015) An empirical model to obtain the kinetic parameters of lignocellulosic biomass pyrolysis in an independent parallel reactions scheme. Fuel Process Technol 140:222–230. https://doi.org/10.1016/j.fuproc.2015.09.001
Santos JCO, Souza AG (2007) Kinetic parameters on thermal degradation of edible vegetable oils by thermogravimetric Data.pdf. J Eng Appl Sci 2:501–503
Shadangi KP, Mohanty K (2014) Kinetic study and thermal analysis of the pyrolysis of non-edible oilseed powders by thermogravimetric and differential scanning calorimetric analysis. Renew Energy 63:337–344. https://doi.org/10.1016/j.renene.2013.09.039
Silvestre WP, Galafassi PL, Ferreira SD, Godinho M, Pauletti GF, Baldasso C (2018a) Fodder radish seed cake biochar for soil amendment. Environ Sci Pollut Res 25:25143–25154. https://doi.org/10.1007/s11356-018-2571-4
Silvestre WP, Pauletti GF, Godinho M, Baldasso C (2018b) Fodder radish seed cake pyrolysis for bio-oil production in a rotary kiln reactor. Chem Eng Process Process Intensif 124:235–244. https://doi.org/10.1016/j.cep.2017.12.020
Slopiecka K, Bartocci P, Fantozzi F (2012) Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy 97:491–497. https://doi.org/10.1016/j.apenergy.2011.12.056
Smets K, Adriaensens P, Reggers G, Schreurs S, Carleer R, Yperman J (2011) Flash pyrolysis of rapeseed cake: influence of temperature on the yield and the characteristics of the pyrolysis liquid. J Anal Appl Pyrolysis 90:118–125. https://doi.org/10.1016/j.jaap.2010.11.002
Sokoto MA, Singh R, Krishna BB, Kumar J, Bhaskar T (2016) Non-isothermal kinetic study of de-oiled seeds cake of African star apple (Chrosophyllum albidum) using thermogravimetry. Heliyon 2:e00172. https://doi.org/10.1016/j.heliyon.2016.e00172
Stedile T, Ender L, Meier HF, Simionatto EL, Wiggers VR (2015) Comparison between physical properties and chemical composition of bio-oils derived from lignocellulose and triglyceride sources. Renew Sustain Energy Rev 50:92–108. https://doi.org/10.1016/j.rser.2015.04.080
TAPPI (2006) T222 om-02. Acid-insoluble lignin in wood and pulp. TAPPI test methods. Tappi Press, Atlanta. https://doi.org/10.1016/j.biortech.2006.08.008
Tappi (2007) Solvent extractives of wood and pulp (proposed revision of T 204cm-97). Tappi, Atlanta. https://doi.org/10.1023/a:1019003230537
Tiptipakorn S, Damrongsakkul S, Ando S, Hemvichian K, Rimdusit S (2007) Thermal degradation behaviors of polybenzoxazine and silicon-containing polyimide blends. Polym Degrad Stab 92:1265–1278. https://doi.org/10.1016/j.polymdegradstab.2007.03.021
Ucar S, Ozkan AR (2008) Characterization of products from the pyrolysis of rapeseed oil cake. Bioresour Technol 99:8771–8776. https://doi.org/10.1016/j.biortech.2008.04.040
Viju D, Gautam R, Vinu R (2018) Application of the distributed activation energy model to the kinetic study of pyrolysis of Nannochloropsis oculata. Algal Res 35:168–177. https://doi.org/10.1016/j.algal.2018.08.026
Vyazovkin S (1993) An approach to the solution of the inverse kinetic problem in the case of complex processes. Thermochim Acta 223:201–206. https://doi.org/10.1016/0040-6031(93)80135-W
Vyazovkin S (2001) Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem 22:178–183. https://doi.org/10.1002/1096-987X(20010130)22:2%3c178:AID-JCC5%3e3.0.CO;2-%23
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ (2014) ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta 590:1–23. https://doi.org/10.1016/j.tca.2014.05.036
Wako FM, Reshad AS, Goud VV (2018) Thermal degradation kinetics study and thermal cracking of waste cooking oil for biofuel production. J Therm Anal Calorim 131:2157–2165. https://doi.org/10.1007/s10973-017-6760-z
Wang H, Yang J, Long S, Wang X, Yang Z, Li G (2004) Studies on the thermal degradation of poly(phenylene sulfide sulfone). Polym Degrad Stab 83:229–235. https://doi.org/10.1016/S0141-3910(03)00266-0
Wei X, Ma X, Peng X, Yao Z, Yang F, Dai M (2018) Comparative investigation between co-pyrolysis characteristics of protein and carbohydrate by TG-FTIR and Py-GC/MS. J Anal Appl Pyrolysis 135:209–218. https://doi.org/10.1016/j.jaap.2018.08.031
WHO (2004) Toxic oil syndrome: ten years of progress. WHO, Geneva
Xin Y, Cao H, Yuan Q, Wang D, Liu Y (2018) Kinetic analysis of cattle manure pyrolysis process with a novel two-step method: pseudo-component model coupled with multipeak gaussian fitting. Environ Prog Sustain Energy 37:1618–1625. https://doi.org/10.1002/ep.12843
Zabaniotou A, Ioannidou O, Skoulou V (2008) Rapeseed residues utilization for energy and 2nd generation biofuels. Fuel 87:1492–1502. https://doi.org/10.1016/j.fuel.2007.09.003
Acknowledgements
The authors would like to acknowledge the National Council for Scientific and Technological Development (CNPq No. 161524/2015-0) for providing the scholarships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Junges, J., Silvestre, W.P., De Conto, D. et al. Non-isothermal kinetic study of fodder radish seed cake pyrolysis: performance of model-free and model-fitting methods. Braz. J. Chem. Eng. 37, 139–155 (2020). https://doi.org/10.1007/s43153-020-00023-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00023-z