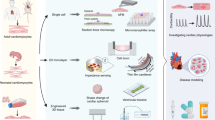
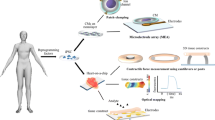
Abstract
Recently, organ-on-chips have become a fast-growing research field with the widespread development of microfluidic chips and synthetic materials in tissue engineering. Due to the existing cardiotoxicity of many cardiovascular drugs, heart-on-chips which are promising to replace traditional animal models have been extensively researched and developed to mimic human organ functions in vitro. The heart-on-chips mainly focus on cardiac mechanics, which is regarded as the central indicator of in vitro heart models and drug testing. Traditional methods for the detection of myocardial mechanics have been demonstrated complex and inefficient in heart-on-chips. Therefore, photonic crystal materials with unique optical properties have attracted interests and have been introduced into the heart-on-chips, developing a visualized self-reporting system for cardiomyocytes activity monitoring. In this review, photonic crystal-based heart-on-chips for biosensing are introduced, as well as the fabrication methods and design criteria of them. The characterizations of the photonic crystal materials are classified into optical properties and structural properties, and their applications in cell culture and biosensing are further discussed. Then, several representative examples and developments of the integration of photonic crystal materials into microfluidic chips are described in detail. Finally, potentials and limitations are put forward to promote the development of the photonic crystal-based intelligent heart-on-chips.













Similar content being viewed by others
References
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668. https://doi.org/10.1126/science.1188302
Huh D, Fujioka H, Tung YC, Futai N, Paine R, Grotberg JB, Takayama S (2007) Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci USA 104:18886–18891. https://doi.org/10.1073/pnas.0610868104
Huh D, Leslie DC, Matthews BD et al (2012) A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4:159ra147. https://doi.org/10.1126/scitranslmed.3004249
Perestrelo AR, Águas AC, Rainer A, Forte G (2015) Microfluidic organ/body-on-a-chip devices at the convergence of biology and microengineering. Sensors 15:31142–31170. https://doi.org/10.3390/s151229848
Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Science 364:960–965. https://doi.org/10.1126/science.aaw7894
Piccini JP, Whellan DJ, Berridge BR et al (2009) Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the critical path initiative. Am Heart J 158:317–326. https://doi.org/10.1016/j.ahj.2009.06.007
Shah RR (2006) Can pharmacogenetics help rescue drugs withdrawn from the market? Future Med. https://doi.org/10.2217/14622416.7.6.889
Kurokawa YK, George SC (2016) Tissue engineering the cardiac microenvironment: multicellular microphysiological systems for drug screening. Adv Drug Deliv Rev 96:225–233. https://doi.org/10.1016/j.addr.2015.07.004
Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE (2015) Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 5:8883. https://doi.org/10.1038/srep08883
Yesil-Celiktas O, Hassan S, Miri AK, Maharjan S, Al-kharboosh R, Quiñones-Hinojosa A, Zhang YS (2018) Mimicking human pathophysiology in organ-on-chip devices. Adv Biosyst 2:1800109. https://doi.org/10.1002/adbi.201800109
Zhang YS, Aleman J, Shin SR et al (2017) Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci USA 114:E2293–E2302. https://doi.org/10.1073/pnas.1612906114
Shin SR, Kilic T, Zhang YS et al (2017) Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell Secretomes. Adv Sci 4:1600522. https://doi.org/10.1002/advs.201600522
Boudou T, Legant WR, Mu A et al (2012) A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A 18:910–919. https://doi.org/10.1089/ten.tea.2011.0341
Grosberg A, Alford PW, McCain ML, Parker KK (2011) Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 11:4165–4173. https://doi.org/10.1039/C1LC20557A
Wang L, Dou W, Malhi M et al (2018) Microdevice platform for continuous measurement of contractility, beating rate, and beating rhythm of human-induced pluripotent stem cell-cardiomyocytes inside a controlled incubator environment. ACS Appl Mater Interfaces 10:21173–21183. https://doi.org/10.1021/acsami.8b05407
Polacheck WJ, Chen CS (2016) Measuring cell-generated forces: a guide to the available tools. Nat Methods 13:415. https://doi.org/10.1038/nmeth.3834
Lind JU, Busbee TA, Valentine AD et al (2017) Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater 16:303–308. https://doi.org/10.1038/nmat4782
Yoon J, Eyster TW, Misra AC, Lahann J (2015) Cardiomyocyte-driven actuation in biohybrid microcylinders. Adv Mater 27:4509–4515. https://doi.org/10.1002/adma.201501284
Kang Y, Walish JJ, Gorishnyy T, Thomas EL (2007) Broad-wavelength-range chemically tunable block-copolymer photonic gels. Nat Mater 6:957–960. https://doi.org/10.1038/nmat2032
Ge D, Lee E, Yang L, Cho Y, Li M, Gianola DS, Yang S (2015) A robust smart window: reversibly switching from high transparency to angle-independent structural color display. Adv Mater 27:2489–2495. https://doi.org/10.1002/adma.201500281
Yue Y, Kurokawa T, Haque MA et al (2014) Mechano-actuated ultrafast full-colour switching in layered photonic hydrogels. Nat Commun 5:1–8. https://doi.org/10.1038/ncomms5659
Phillips KR, England GT, Sunny S, Shirman E, Shirman T, Vogel N, Aizenberg J (2016) A colloidoscope of colloid-based porous materials and their uses. Chem Soc Rev 45:281–322. https://doi.org/10.1039/C5CS00533G
Yang D, Ye S, Ge J (2014) From metastable colloidal crystalline arrays to fast responsive mechanochromic photonic gels: an organic gel for deformation-based display panels. Adv Funct Mater 24:3197–3205. https://doi.org/10.1002/adfm.201303555
Kolle M, Zheng B, Gibbons N, Baumberg JJ, Steiner U (2010) Stretch-tuneable dielectric mirrors and optical microcavities. Opt Exp 18:4356–4364. https://doi.org/10.1364/OE.18.004356
Park TH, Yu S, Cho SH et al (2018) Block copolymer structural color strain sensor. NPG Asia Mater 10:328–339. https://doi.org/10.1038/s41427-018-0036-3
Diao YY, Liu XY, Toh GW, Shi L, Zi J (2013) Multiple structural coloring of silk-fibroin photonic crystals and humidity-responsive color sensing. Adv Funct Mater 23:5373–5380. https://doi.org/10.1002/adfm.201203672
Yablonovitch E (1987) Inhibited spontaneous emission in solid-state physics and electronics. Phys Rev Lett 58:2059. https://doi.org/10.1103/PhysRevLett.58.2059
John S (1987) Strong localization of photons in certain disordered dielectric superlattices. Phys Rev Lett 58:2486. https://doi.org/10.1103/PhysRevLett.58.2486
Sun J, Bhushan B, Tong J (2013) Structural coloration in nature. RSC Adv 3:14862–14889. https://doi.org/10.1039/C3RA41096J
Cong H, Yu B, Tang J, Li Z, Liu X (2013) Current status and future developments in preparation and application of colloidal crystals. Chem Soc Rev 42:7774–7800. https://doi.org/10.1039/C3CS60078E
Zhao Y, Xie Z, Gu H, Zhu C, Gu Z (2012) Bio-inspired variable structural color materials. Chem Soc Rev 41:3297–3317. https://doi.org/10.1039/c2cs15267c
Davis KE, Russel WB, Glantschnig WJ (1991) Settling suspensions of colloidal silica: observations and X-ray measurements. J Chem Soc, Faraday Trans 87:411–424. https://doi.org/10.1039/FT9918700411
Denkov ND, Velev OD, Kralchevsky PA, Ivanov IB, Yoshimura H, Nagayama K (1993) Two-dimensional crystallization. Nature 361:26. https://doi.org/10.1038/361026a0
Jiang P, Bertone JF, Hwang KS, Colvin VL (1999) Single-crystal colloidal multilayers of controlled thickness. Chem Mater 11:2132–2140. https://doi.org/10.1021/cm990080+
Yang H, Jiang P (2010) Large-scale colloidal self-assembly by doctor blade coating. Langmuir 26:13173–13182. https://doi.org/10.1021/la101721v
Gu ZZ, Fujishima A, Sato O (2002) Fabrication of high-quality opal films with controllable thickness. Chem Mater 14:760–765. https://doi.org/10.1021/cm0108435
Zhao Y, Shang L, Cheng Y, Gu Z (2014) Spherical colloidal photonic crystals. Acc Chem Res 47:3632–3642. https://doi.org/10.1021/ar500317s
Zhao Y, Zhao X, Sun C, Li J, Zhu R, Gu Z (2008) Encoded silica colloidal crystal beads as supports for potential multiplex immunoassay. Anal Chem 80:1598–1605. https://doi.org/10.1021/ac702249a
Wang JT, Wang J, Han JJ (2011) Fabrication of advanced particles and particle—based materials assisted by droplet—based microfluidics. Small 7:1728–1754. https://doi.org/10.1002/smll.201001913
Kim SH, Jeon SJ, Yi GR, Heo CJ, Choi JH, Yang SM (2008) Optofluidic assembly of colloidal photonic crystals with controlled sizes, shapes, and structures. Adv Mater 20:1649–1655. https://doi.org/10.1002/adma.200703022
Ge J, Lee H, He L et al (2009) Magnetochromatic microspheres: rotating photonic crystals. J Am Chem Soc 131:15687–15694. https://doi.org/10.1021/ja903626h
Srinivas RL, Chapin SC, Doyle PS (2011) Aptamer-functionalized microgel particles for protein detection. Anal Chem 83:9138–9145. https://doi.org/10.1021/ac202335u
Kanai T, Lee D, Shum HC, Weitz DA (2010) Fabrication of tunable spherical colloidal crystals immobilized in soft hydrogels. Small 6:807–810. https://doi.org/10.1002/smll.200902314
Xu Y, Wang H, Chen B, Liu H, Zhao Y (2019) Emerging barcode particles for multiplex bioassays. Sci China Mater 62:89–324. https://doi.org/10.1007/s40843-018-9330-5
Choi SW, Zhang Y, Thomopoulos S, Xia Y (2010) In vitro mineralization by preosteoblasts in poly (DL-lactide-co-glycolide) inverse opal scaffolds reinforced with hydroxyapatite nanoparticles. Langmuir 26:12126–12131. https://doi.org/10.1021/la101519b
Zhang YS, Regan KP, Xia Y (2013) Controlling the pore sizes and related properties of inverse opal scaffolds for tissue engineering applications. Macromol Rapid Commun 34:485–491. https://doi.org/10.1002/marc.201200740
Choi SW, Xie J, Xia Y (2009) Chitosan-based inverse opals: three-dimensional scaffolds with uniform pore structures for cell culture. Adv Mater 21:2997–3001. https://doi.org/10.1002/adma.200803504
Zhang YS, Zhu C, Xia Y (2017) Inverse opal scaffolds and their biomedical applications. Adv Mater 29:1701115. https://doi.org/10.1002/adma.201701115
Wang J, Chen G, Zhao Z, Sun L, Zou M, Ren JA, Zhao Y (2018) Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci China Mater 61:1314–1324. https://doi.org/10.1007/s40843-018-9251-9
He J, Sun C, Gu Z et al (2018) Morphology, migration, and transcriptome analysis of schwann cell culture on butterfly wings with different surface architectures. ACS Nano 12:9660–9668. https://doi.org/10.1021/acsnano.8b00552
Elbaz A, Lu J, Gao B, Zheng F, Mu Z, Zhao Y, Gu Z (2017) Chitin-based anisotropic nanostructures of butterfly wings for regulating cells orientation. Polymers 9:386. https://doi.org/10.3390/polym9090386
Dong C, Lv Y (2016) Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers 8:42. https://doi.org/10.3390/polym8020042
Dvir T, Timko BP, Kohane DS, Langer R (2011) Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6:13. https://doi.org/10.1038/nnano.2010.246
Engel E, Michiardi A, Navarro M, Lacroix D, Planell JA (2008) Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol 26:39–47. https://doi.org/10.1016/j.tibtech.2007.10.005
Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL (2010) The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers 2:522–553. https://doi.org/10.3390/polym2040522
Ding Y, Sun J, Ro HW et al (2011) Thermodynamic underpinnings of cell alignment on controlled topographies. Adv Mater 23:421–425. https://doi.org/10.1002/adma.201001757
Lee YS, Livingston AT (2011) Electrospun nanofibrous materials for neural tissue engineering. Polymers 3:413–426. https://doi.org/10.3390/polym3010413
Schulte VA, Díez M, Möller M, Lensen MC (2009) Surface topography induces fibroblast adhesion on intrinsically nonadhesive poly (ethylene glycol) substrates. Biomacromol 10:2795–2801. https://doi.org/10.1021/bm900631s
Lu H, Feng Z, Gu Z, Liu C (2009) Growth of outgrowth endothelial cells on aligned PLLA nanofibrous scaffolds. J Mater Sci Mater Med 20:1937–1944. https://doi.org/10.1007/s10856-009-3744-y
Zhang Z, Cui H (2012) Biodegradability and biocompatibility study of poly (chitosan-g-lactic acid) scaffolds. Molecules 17:3243–3258. https://doi.org/10.3390/molecules17033243
Park S, Park KM (2016) Engineered polymeric hydrogels for 3D tissue models. Polymers 8:23. https://doi.org/10.3390/polym8010023
Wang YC, Tang ZM, Feng ZQ, Xie ZY, Gu ZZ (2010) Stretched inverse opal colloid crystal substrates-induced orientation of fibroblast. Biomed Mater 5:035011. https://doi.org/10.1088/1748-6041/5/3/035011
Lu J, Zou X, Zhao Z, Mu Z, Zhao Y, Gu Z (2015) Cell orientation gradients on an inverse opal substrate. ACS Appl Mater Interfaces 7:10091–10095. https://doi.org/10.1021/acsami.5b02835
Fattahi P, Borhan A, Abidian MR (2013) Microencapsulation: microencapsulation of chemotherapeutics into monodisperse and tunable biodegradable polymers via electrified liquid jets: control of size, shape, and drug release. Adv Mater 25:4529. https://doi.org/10.1002/adma.201370205
Zhang B, Cheng Y, Wang H, Ye B, Shang L, Zhao Y, Gu Z (2015) Multifunctional inverse opal particles for drug delivery and monitoring. Nanoscale 7:10590–10594. https://doi.org/10.1039/C5NR02324F
Zhang H, Liu D, Wang L et al (2017) Microfluidic encapsulation of prickly zinc-doped copper oxide nanoparticles with VD1142 modified spermine acetalated dextran for efficient cancer therapy. Adv Healthc Mater 6:1601406. https://doi.org/10.1002/adhm.201601406
Zhang H, Liu Y, Wang J, Shao C, Zhao Y (2019) Tofu-inspired microcarriers from droplet microfluidics for drug delivery. Sci China Chem 62:87–94. https://doi.org/10.1007/s11426-018-9340-y
Liu Y, Shao C, Bian F, Yu Y, Wang H, Zhao Y (2018) Egg component-composited inverse opal particles for synergistic drug delivery. ACS Appl Mater Interfaces 10:17058–17064. https://doi.org/10.1021/acsami.8b03483
Ueno K, Matsubara K, Watanabe M, Takeoka Y (2007) An electro-and thermochromic hydrogel as a full-color indicator. Adv Mater 19:2807–2812. https://doi.org/10.1002/adma.200700159
Wang T, Liu J, Nie F (2018) Non-dye cell viability monitoring by using pH-responsive inverse opal hydrogels. J Mater Chem B 6:1055–1065. https://doi.org/10.1039/C7TB02631E
Chen Z, Fu F, Yu Y, Wang H, Shang Y, Zhao Y (2019) Cardiomyocytes-actuated Morpho butterfly wings. Adv Mater 31:1805431. https://doi.org/10.1002/adma.201805431
Fu F, Shang L, Chen Z, Yu Y, Zhao Y (2018) Bioinspired living structural color hydrogels. Sci Robot 3:eaar8580. https://doi.org/10.1126/scirobotics.aar8580
Li LJ, Chen ZY, Shao CM, Sun LY, Sun LY, Zhao YJ (2020) Graphene hybrid anisotropic structural color film for cardiomyocytes’ monitoring. Adv Funct Mater 30:1906353. https://doi.org/10.1002/adfm.201906353
Shang Y, Chen Z, Fu F et al (2018) Cardiomyocyte-driven structural color actuation in anisotropic inverse opals. ACS Nano 13:796–802. https://doi.org/10.1021/acsnano.8b08230
Choi TM, Park JG, Kim YS, Manoharan VN, Kim SH (2015) Osmotic-pressure-mediated control of structural colors of photonic capsules. Chem Mater 27:1014–1020. https://doi.org/10.1021/cm5043292
Shang L, Cheng Y, Zhao Y (2017) Emerging droplet microfluidics. Chem Rev 117:7964–8040. https://doi.org/10.1021/acs.chemrev.6b00848
Wang H, Gu H, Chen Z, Shang L, Zhao Z, Gu Z, Zhao Y (2017) Enzymatic inverse opal hydrogel particles for biocatalyst. ACS Appl Mater Interfaces 9:12914–12918. https://doi.org/10.1021/acsami.7b01866
Wang H, Liu Y, Chen Z, Sun L, Zhao Y (2020) Anisotropic structural color particles from colloidal phase separation. Sci Adv 6:eaay1438. https://doi.org/10.1126/sciadv.aay1438
Sun L, Chen Z, Bian F, Zhao Y (2020) Bioinspired soft robotic caterpillar with cardiomyocyte drivers. Adv Funct Mater 30:1907820. https://doi.org/10.1002/adfm.201907820
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 61927805), the Natural Science Foundation of Jiangsu (Grant No. BE2018707) and the Scientific Research Foundation of Nanjing University and Drum Tower Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This review does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shang, Y., Chen, Z., Zhang, Z. et al. Heart-on-chips screening based on photonic crystals. Bio-des. Manuf. 3, 266–280 (2020). https://doi.org/10.1007/s42242-020-00073-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-020-00073-9