Abstract
Geothermal energy provides an important resource in Antarctic marine ecosystems, exemplified by the recent discovery of large-sized chemosymbiotic vesicomyid bivalves (subfamily Pliocardiinae) in the Southern Ocean. These clams, which we identified as Archivesica s.l. puertodeseadoi, have been reported as dead shells in areas previously covered by Larsen A and B ice shelves (eastern Antarctic Peninsula) and as live animals from active hydrothermal sites in the Kemp Caldera (South Sandwich Arc) at depths of 852–1487 m. Before, A. puertodeseadoi was known only from its type locality in the Argentine Sea, so we considerably extend the range of the species. Observations taken by remotely operated vehicle (ROV) footage show that the clams can live buried in sediment, or epilithically on the surface of rocks in diffuse geothermal flow. Experimental respirometry was conducted at surface pressure on individual bivalves acclimated to either their habitat temperature (4 °C) or elevated temperature (10 °C). The range of standard metabolic rates, from 3.13 to 6.59 (MO2, μmol O2 h−1 g−1 dry tissue mass), is similar to rates measured ex situ for other species in this clade, and rates did not differ significantly between temperature groups. Taken together, these data indicate a range of ecophysiological flexibility for A. puertodeseadoi. Although adapted to a specialist mode of life, this bivalve exploits a relatively broad range of habitats in the Southern Ocean: within sulphidic sediments, epilithically in the presence of diffuse sulphidic flow, or in deep methane-enriched seawater trapped under ice.
Similar content being viewed by others
Introduction
Deep-sea chemosynthetic ecosystems were first discovered in 1977 around hydrothermal vents on the Galapagos Rift, starting with a surprising observation of dense assemblages of giant vesicomyid bivalves (Corliss et al. 1979). A few years after the discovery of hydrothermal vents, similar lush communities including vesicomyid beds were also found at deep-sea hydrocarbon seeps (Paull et al. 1984; Hecker 1985) and from organic falls (Baco et al. 1999; Cosel and Olu 2009). These chemosynthesis-based habitats were unlike anything then known from the deep sea, complementing the idea of an empty abyss with a network of self-sustaining oases drawing energy from reduced chemical compounds. Chemosynthesis-based ecosystems are now known from all oceans, including the Southern Ocean, and chemosymbiotic vesicomyid bivalves dominate many of these communities (Giere et al. 2003; Levin 2005).
The family Vesicomyidae is distributed worldwide at 100–10,730 m deep (Krylova et al. 2018) and contains two subfamilies: Pliocardiinae and Vesicomyinae (Krylova and Sahling 2010; Johnson et al. 2017). The pliocardiines occur only in sulphide-rich environments and form an evolutionary radiation dependent on chemosymbiotic microbes (Krylova and Sahling 2010; Johnson et al. 2017). All species in this subfamily harbour chemoautotrophic sulphur-oxidizing symbiotic bacteria in their gills, transmitted vertically from parent to offspring (Ikuta et al. 2016), and apparently derive most or all of their nutrition from this association (e.g. Fisher et al. 1988; Dubilier et al. 2008). Pliocardiines include medium- and large-sized molluscs, the largest of which can reach nearly 30 cm in length (Krylova and Sahling 2010). In contrast, members of the sister-group Vesicomyinae are characteristically small, with shell lengths generally under 10 mm, live in a broad range of deep-sea settings preferring biotopes with high amounts of organic matter and are apparently exclusively filter-feeders (Krylova et al. 2015, 2018). Data on stable carbon and nitrogen isotopic composition of four vesicomyine species do not suggest chemotrophic origin of carbon in their nutrition (Krylova et al. 2018). The contrasting body size in the two subfamilies follows a trend seen in other groups of molluscs where the evolution of chemoautotrophic bacterial symbiosis is associated with increasing body size (Taylor and Glover 2010; Vermeij 2016).
Despite the wide distribution of the family, it was represented in the Southern Ocean only by two described non-chemosymbiotic vesicomyine species, Vesicomya sirenkoi (Egorova 1998) reported from 1121 to 6348 m in the Amundsen, Scotia, and Weddell Seas (Linse 2004, 2014) and Vesicomya laevis (Pelseneer 1903) distributed east and west of the Antarctic Peninsula at the depths of 378–4572 m (Allen 2001). There is also another as yet undescribed species Vesicomya sp. from the abyssal Weddell Sea (Krylova and Sahling 2010). The chemosymbiotic subfamily Pliocardiinae has been observed several times in the Southern Ocean but usually without clear records of live animals that would illuminate the local ecological setting. Dead valves were reported from the Ross Sea without species-level identification (Marshall and Tracey 2015). Evidence of pliocardiine clams had also been observed at cold seep areas in the Weddell Sea, from sites previously covered by the collapsed ice shelves Larsen A & B at about 850 m (Gutt 2008). These Weddell Sea sites were characterised by extensive bacterial mats and patches of shells (Domack et al. 2005), later attributed to Calyptogena sp. (Niemann et al. 2009). Although found several times in the same area, it was not clear whether any of the earlier records actually included live bivalves, and later only dead valves were collected by dredging (Gutt 2008; Knust 2012).
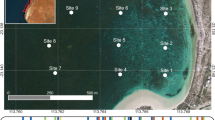
Diverse geofluid-associated habitats have now been described from the Southern Ocean including active hydrothermal vents at the East Scotia Ridge (ESR), which represent a separate vent biogeographic province distinct from other known vents (Rogers et al. 2012). Furthermore, the Kemp Caldera (59°42′S 28°20′W) located west of the Kemp Seamount on the South Sandwich Arc (Leat et al. 2013, 2016) also hosts an additional hydrothermal vent fauna closely connected to the ESR vents (Roterman et al. 2016; Linse et al. 2019a). The sill depth is at 900 m, while the inner caldera floor depth is ~ 1600 m and has a resurgent cone in its centre. Hydrothermal activity was reported on the eastern side of the resurgent cone, originating from a white smoking vent field and with recognisable signatures in the water column (Cole et al. 2014; Hawkes et al. 2014). In the vicinity of the resurgent cone, nine different small-scale hydrothermally active sites are present and four were dominated by large pliocardiine clams, including the first live records of the group for the Antarctic region (Rogers 2010; Linse et al. 2019a), and these sites are the focus of the present study.
Responses of animals to temperature may be a key factor to their small and broader scale distributions. This is particularly true in the context of polar deep-sea environments where geothermal sites are characterised by extremely steep temperature gradients. For example, the ability to maintain a consistent standard metabolic rate in differing temperatures would be evidence of an ecophysiological flexibility in a relatively broad tolerance for thermal variability. In the present study, we provide new in situ live observations of pliocardiine clams from the Kemp Caldera, and ex situ measurements of its oxygen metabolism at the habitat temperature (4 °C) and in elevated temperature conditions (10 °C), in order to understand its potential habitat occupation across the Antarctic region. A combination of ecological observations, based on well-resolved taxonomy, and evidence from experimental physiology can indicate how small-scale environmental variations influence habitat usage and species distribution.
Material and methods
Seafloor imagery and specimen collection
Pliocardiine bivalves were discovered in the Kemp Caldera in 2010 during expedition JC42 of RRS James Cook using ROV Isis (Fig. 1, Table 1) (Rogers 2010). For seafloor imagery, ROV Isis (Marsh et al. 2013) was equipped with a video camera (Insite Pacific Atlas), a 1080i high-definition video camera (Insite Pacific Mini Zeus), and a 3.3-megapixel stills camera (Insite Scorpio). Two lasers, 10 cm apart, were mounted parallel to the focal axis of the video cameras to provide a size reference. A second expedition to the area in 2019, PS119 on RV Polarstern with ROV MARUM-QUEST provided follow up observations, collections, and additional experimental manipulations (Table 1).
On JC42, specimens were collected by a scoop or the suction sampler and stored in a biobox or suction sampler jar. Live bivalves were fixed in pre-cooled 96% ethanol or 4% formaldehyde solution (formalin) and dead shells were washed and dried. On PS119, specimens were collected in a net stored in a biobox and recovered to the surface alive. Animals were collected on two dives: MARUM QUEST dive 447, n = 16, and dive 448, n = 50. A high temperature lance probe and a CTD sensor on the bottom surface of the ROV were used to measure sediment and water temperatures, within and adjacent to (within 2 m) the sites where the clams lived. On recovery to the surface, live animals were washed with surface seawater to remove sediment, and transferred immediately to seawater aquaria chilled to 4 °C.
Morphology and genetics
In addition to specimens collected in the Kemp Caldera, we studied dead valves obtained in the Larsen B area, Weddell Sea, by RV Polarstern, ANT XXIII/8. For comparative purposes we additionally examined specimens of Laubiericoncha myriamae Cosel and Olu, 2008 and Archivesica s.l. chuni (Thiele and Jaeckel, 1931). Shell morphology and soft part anatomy were examined visually and with an Olympus SZ40 stereo zoom microscope. Shell length and height of Kemp Caldera clams were measured using digital Vernier callipers accurate to 0.1 mm.
A total of eight specimens from the Kemp Caldera were used for genetic analyses, including three epilithic specimens from cobble and basalt bottoms and five infaunal specimens from sedimented seafloor (Fig. 1b). DNA was extracted from foot tissue with the DNeasy Tissue Extraction Kit (QIAGEN, Crawley, U.K.) following the directions of the manufacturer. The primers used were LCO1490 and HCO2198 for the COI gene (Folmer et al. 1994), SSU 1F and SSU 82R for the 18S gene (Medlin et al. 1988), and LSU 5 and LSU 3 for the 28S gene (Littlewood 1994). Amplification of the mitochondrial COI gene was carried out using polymerase chain reaction (PCR) with standard reagents in 25 µL total volume (2.5 µL of 10 × buffer, 0.5 µL of 10 mM dNTPs, 2.5 µL of each 10 µM primer, 0.125 µL of 2.5U Taq DNA Polymerase, 5 µL of ‘Q’ solution, 10.875 µL double-distilled water, 1 µL genomic DNA) and PCR for 18S and 28S genes were performed in 40 µL volumes (containing final concentrations of 1 × PCR buffer (Bioline), 5% bovine serum albumin 10 mg·mL−1 (Sigma), 200 μM each dNTP, 0.5 μM of each primer, 0.5 units of Taq DNA Polymerase (Bioline), and 1 μL template DNA).
The following cycling conditions were used for COI: 94 °C for 2 min, [94 °C for 20 secs, 50 °C for 20 secs and 72 °C for 1–2 min] for 35 cycles and a final extension at 72 °C for 7 min. For 18S the following conditions were used: 94 °C for 2 min, [94 °C for 1 min, 60 °C for 30 secs, 72 °C for 1 min] for 35 cycles; 72 °C for 4 min. For 28S the same conditions were used except that the annealing temperature was set to 45 °C.
PCR purification and DNA sequencing of both forward and reverse strands were carried out by LGC Genomics. Sequences obtained were assembled, checked by eye, and aligned using Geneious R11 (https://www.geneious.com/). Uncorrelated p-distances were calculated among resulting haplotypes in the software MEGA X (Kumar et al. 2018). Sequences generated for the present study were deposited in GenBank under accession numbers MN523628–MN523650.
Experimental physiology
Animals were maintained in plastic aquaria in temperature-controlled laboratory rooms on board RV Polarstern. Two different constant temperature rooms were used, set to 4 °C and 10 °C. All animals were initially held in the 4 °C room for 24 h, and subsequently a subset of approximately half of the available animals were moved in a 50 L volume of 4 °C water into the 10 °C environmental room (air temperature), and experiments at elevated temperature commenced after the aquarium temperature had equilibrated to the higher air temperature (this took approximately 24 h). Temperature measurements in situ taken in 2019 indicated that sediment temperature of an area densely populated by clams was 4.4 °C above the 0.3 °C ambient temperature, which was the main basis for using 4 °C as a ‘habitat’ temperature treatment for physiology experiments.
Individual closed chamber respirometry experiments were used to measure routine oxygen uptake rates, following established methods (Carey et al. 2013; Sigwart and Chen 2018). Each animal was placed inside a sealed, oxygen- and water-tight cylindrical Perspex chamber fitted with a fibre optic oxygen probe (Ocean Optics, Dunedin, Florida). Oxygen concentrations were measured continuously and recorded at 1 s intervals as a percentage of fully saturated oxygen concentration. Probes were calibrated to 100% (fully saturated) concentrations based on aquarium conditions (with aeration from a standard aquarium air pump and mixed with circulating pump), and to 0% in a zero-oxygen solution of sodium nitrate in seawater (1 g 1 L−1).
Available equipment allowed for six simultaneous trials, held in a common waterbath to maintain temperature, and these simultaneous trials always included at least one chamber filled with seawater and no experimental animal to measure background uptake rates as a control. Control consumption per volume were subtracted from measured animal uptake to obtain metabolic rates. A magnetic stir bar was added to each chamber to aid mixing and the whole waterbath was elevated on rockers such that the ship’s motion caused continuous gentle agitation. The experimental volume was measured at the end of the trial by removing the probe, drying the outside of the chamber, emptying the contents into a measuring beaker and then removing the water with a graduated syringe.
Data from each trial were then processed to extract a smooth part of the experiment where oxygen was decreasing steadily. Smooth declining trends in the oxygen values in this type of experiment indicate normal behaviour by the subject and a well mixed water mass inside the chamber. The rate was interpolated from an OLS regression fitted to the extracted portion of the trace and then calculated based on the starting and ending concentrations on the fitted line. These oxygen concentrations were then converted to molar oxygen based on the volume, salinity, and temperature of the individual experiment following Benson and Krause (1984). Changes in molar oxygen over time (consumption rate, VO2, mmol O2 h−1), were then standardised to animal dry mass, to provide standard metabolic rate (MO2, mmol O2 g−1 h−1).
We completed experiments on 27 individual clams, ranging from 18.3 to 104.6 mm shell length (Table 2). Animals were divided into two experimental groups, and measured at temperatures close to the habitat conditions at 4 °C (n = 16, at 3.89 °C ± 1.14 s.d.) or elevated temperature at 10 °C (n = 11, at 10.14 °C ± 0.19 s.d.).
Institutional abbreviations
BAS, British Antarctic Survey, Cambridge.
IORAS, Shirshov Institute of Oceanology, Moscow.
MNHN, Muséum National d’Histoire Naturelle, Paris.
MARUM, Center for Marine Environment Sciences and Faculty of Geosciences, University of Bremen, Bremen.
Results
Habitat conditions of pliocardiines in Kemp Caldera
Living pliocardiine clams were definitively observed for the first time in the Southern Ocean in 2010 at the study sites for the present paper, in two areas and different sites around the resurgent cone in the Kemp Caldera at depths ranging from 1320 to 1487 m (Fig. 1); large quantities of dead shells were also observed. The southern area was characterised by basalt cobbles and rough-edged basalt boulders with no visible sediment-covered areas, and the pliocardiines were living epifaunally on the hard rock (Fig. 2a; Table 1). In 2019, the sites with epifaunal clams were surveyed again but only dead shells were found.
In the northern area of the Kemp Caldera, multiple sites were characterised by sediment-covered areas, in the form of either small areas between basalt cobbles and boulders or wide-ranging sediment coverage. In both 2010 and 2019, pliocardiines were observed at some of these sites either fully buried with the siphons visible, sticking out of the sediment, or half-buried with shell and siphons visible (Fig. 2b,c; Table 1). Temperature measurements taken in 2019 found that one area densely populated by clams had a sediment temperature 4.4 °C above the 0.3 °C ambient temperature, compared to 0.25 °C above ambient in the sediment outside the clam bed. Another measurement found a thermal anomaly 0.3 °C above ambient temperature in the water immediately above a dense aggregation of clams (< 1 m altitude).
The overall allometry of shell length and height followed a consistent pattern across clams living epilithically and infaunally, indicating that there is no overall difference in the change of shape over growth that is characteristic to these habitat types (Fig. 3). Of the three epilithic and five infaunal individuals used for molecular sequencing, partial sequences of COI, 18S, and 28S genes were successfully obtained with the exception of 28S from one infaunal individual. For COI (638 bp), a dominant haplotype was shared across six individuals from both ecotypes, and the other two individuals (one epifaunal and one infaunal) exhibited singleton haplotypes that differed from the dominant haplotype by a single base. The uncorrected p-distances among the haplotypes ranged between 0.0016 and 0.0032. For both 18S (517 bp) and 28S (757 bp), all individuals shared the same haplotype regardless of their ecotype. These results suggest that the two ecotypes are genetically indistinguishable.
a Scatterplot comparing shell length and shell height of Archivesica s.l. puertodeseadoi collected from the Kemp Caldera, as well as the type series collected off Argentina (Signorelli and Pastorino 2015). b Archivesica s.l. puertodeseadoi from the Kemp Caldera, infaunal specimen collected 2010, cruise JC42, length = 111.12 cm; right inner valve, with pallial scars highlighted (photo by Heiko Sahling)
Experimental physiology
Although a range of animal sizes was used in experiments, there was only a weak positive correlation of size and metabolic rate (VO2: μmol O2 h−1) in either group (4 °C: R2 = 0.004, p = 0.78; 10 °C: R2 = 0.39, p = 0.02) insufficient to determine a robust estimate of a metabolic scaling exponent. The mean metabolic rate (VO2) did not significantly differ between the 4 °C and 10 °C treatment groups (Mann–Whitney U test, W = 129, p = 0.99). The mean mass-specific metabolic rate (MO2: μmol O2 h−1 g−1 dry tissue mass) was much higher in the 4 °C group (6.59 μmol O2 h−1 g−1 dry tissue mass) than in the 10 °C group (3.13 μmol O2 h−1 g−1 dry tissue mass); however this difference was attributable to higher metabolic rates in the smallest individuals sampled in the 4 °C group and the rates across the two treatments were not statistically significantly different (W = 115, p = 0.60; Fig. 4).
Animals kept in shipboard aquaria remained active and responsive for several days (video provided in Online Resource 1), but after 2–3 days began to deteriorate, the foot and siphons were less frequently extended and the foot appeared paler in colour. Animals remained alive for up to 5 days, and would respond to handling by rapidly clamping shut and ejecting water from the siphon.
Species identification
We identified the pliocardiine species from Kemp Caldera and Larson B as conspecifics of a species previously known only from the South Atlantic on the Argentinian continental slope off Buenos Aires in 877–2204 m depth and originally described as Laubiericoncha puertodeseadoi (Signorelli and Pastorino 2015) based on the Argentinian material (Signorelli and Pastorino 2015). This is also supported by similar shell length to height ratios across sizes between the type series and clams collected from the Southern Ocean (Fig. 3). The Southern Ocean records considerably extend the known geographic range of this species. While the depths of the Kemp Caldera records were within the known bathymetric range, additional Larsen B specimens were collected at a shallower depth of 852 m. Morphological comparisons with closely related species indicated that this species should be assigned to Archivesica sensu lato, with more details presented in the following section.
Systematics
Class BIVALVIA Linnaeus 1758.
Subclass HETERODONTA Neumayr 1884.
Order VENERIDA Gray 1854.
Superfamily GLOSSOIDEA Gray, 1847.
Family VESICOMYIDAE Dall et Simpson, 1901.
Subfamily PLIOCARDIINAE Woodring, 1925.
Archivesica Dall, 1908 sensu lato.
Archivesica s.l. puertodeseadoi (Signorelli et Pastorino, 2015).
Laubiericoncha puertodeseadoi Signorelli and Pastorino 2015: 352, Figs. 1–28.
Archivesica puertodeseadoi – Hansen et al. 2017: 272.
Type locality
Southwestern Atlantic Ocean, off Buenos Aires province coast (37°54.206′S, 54°2.616′W), 2419.59 m; RV “Puerto Deseado”, station 24, bottom trawl, 14 Aug 2012 (Signorelli and Pastorino 2015).
Distribution
Off Buenos Aires province coast, Argentina, southwestern Atlantic Ocean in 877–2,204 m depth (Signorelli and Pastorino 2015); Kemp Caldera, South Sandwich Arc in 1320–1487 m depth; Larsen B area, Weddell Sea, 852 m depth.
Material examined
Archivesica s.l. puertodeseadoi: RRS James Cook cruise 42, ROV Isis, Scotia Sea, South Sandwich Arc, Kemp Caldera, 59°41.677 S, 28°21.086 W, 1320–1487 m, 2010, many specimens with soft parts (BAS, IORAN); RV Polarstern cruise ANT XXIII/8, western Weddell Sea, Larsen B area, 65°26′S, 61°26.5′W, 852 m, 15 January 2007, 2 dry valves and fragments (MARUM).
Other species
Laubiericoncha myriamae: DIAPISUB stn PL DS 03/1, Barbados accretionary prism, site Orénoque A, 10°20.27′N, 58°53.73′W, 1730 m, paratype, 1 specimen with soft parts (MNHN 20,551); R/V Ronald H. Brown, ROV Jason II dive 283, Gulf of Mexico, 21˚N, 91˚ W, 2276–2530 m, 2 July 2007, 3 specimens with soft bodies (IORAN); DSV Alvin dive 3917, Gulf of Mexico, 27°14.02 N, 85°36.66 W, 3234 m, 13 October 2003, 50 articulated dry shells (IORAN).
Archivesica s.l. chuni: BIOZAIRE 3 cruise, RV Atalante, REGAB site, West of Congo river mouth, 5°46.89S, 9°44.65E, 3113–3159 m, 2 January 2004, 2 dry valves (IORAN).
Remarks
Specimens from the Kemp Caldera were consistent with the type material of A. s.l. puertodeseadoi (Signorelli & Pastorino 2015) in the following diagnostic features: oval-elongated valves with slightly tapering posterior margins and 1–2 shallow sulci in dorsal-posterior area, a similar hinge margin with subumbonal pit, the strongest tooth being 1 on the right valve and 2b on the left valve, a pallial line originating from the postero-ventral margin of the anterior adductor scar with nearly triangular pallial sinus and going slightly behind the anterior margin of the posterior adductor scar.
Comparison of the Kemp Caldera material with the type species of Laubiericoncha, Laubiericoncha myriamae Cosel and Olu 2008, based on a paratype specimen as well as additional material, revealed that in spite of the obvious similarity in the general shell outline and the hinge margin configuration, the newly collected specimens differed from L. myriamae in several important characters. These include exhibiting a subumbonal pit in the hinge margin, pallial line lacking in ventral prolongation posteriorly at the end of pallial sinus, and the interlamellar septae in the gills lacking in tubular structure. Furthermore, the inhalant siphon valve only carry short papillae along its margin, in sharp contrast with L. myriamae where the entire surface of valve is covered by conspicuous papillae, a unique feature among known pliocardiines. The inner surface of the inhalant siphon of L. myriamae is also covered by rather large papillae, which were absent in the Southern Ocean clams. On the basis of these differences, we consider that A. s.l. puertodeseadoi and L. myriamae are not congeneric species.
We further compared the Kemp Caldera clams with Archivesica s.l. chuni and Archivesica gigas, the type species of the genus Archivesica s.s.. We found that A. s.l. chuni and A. s.l. puertodeseadoi share morphological features in the abovementioned key characters that differentiate them from L. myriamae, including the same structure of gill interlamellar septae lacking tubes, having similar vulve on the inhalant siphon, and a pallilal line lacking posterior extension. Additionally, both species, A. s.l. chuni and A. s.l. puertodeseadoi, differ from A. gigas by longer syphons and triangular pallial sinuses. According to the molecular results, A. s.l. chuni was nested in a well-defined unnamed subclade, sister to a subclade Archivesica s.s. containing A. gigas (Johnson et al. 2017). So, here for A. s.l. chuni and A. s.l. puertodeseadoi we provisionally use the genus name Archivesica in the wide sense (sensu lato).
In the Kemp Caldera A. s.l. puertodeseadoi was reported in several hard rock and soft sediment habitats (Linse et al. 2019a) which dominant or visually distinct fauna comprised the seastar Paulasterias tyleri, the limpets Cocculina enigmadonta and Lepetodrilus concentricus, the pyconogonids Sericosura bamberi, S. curva, and S. dimorpha, and actinosolid anemones (Mah et al. 2015; Linse et al. 2019b; Chen and Linse 2020).
Notes on Laubiericoncha
Taxonomy of Pliocardiinae is still far from being stabilised, with shell characters used in early taxonomic accounts often failing to provide sufficient discrimination among genera. Including soft anatomical traits has considerably improved the understanding of the taxonomy at the generic level (Cosel and Salas 2001; Krylova and Sahling 2006; Krylova et al. 2014) and recent studies combining soft part morphology and multi-gene molecular analyses have clarified some genus-level characteristics (Johnson et al. 2017). Nevertheless, there are still unresolved problems at both genus- and species-levels due to a high degree of morphological variability, with a number of cryptic and synonymous species revealed in the group by molecular approaches (Audzijonyte et al. 2012; Decker et al. 2012). In these respects, the pliocardiine species from the Kemp Caldera is of particular interest, as it demonstrates a wide range of morphological variability and ecological plasticity.
Laubiericoncha myriamae and A. s.l chuni were initially both placed in Laubiericoncha (Cosel and Olu 2008; Signorelli and Pastorino 2015), but molecular phylogeny indicated that they were divergent at genus level (Decker et al. 2012; Johnson et al. 2017). At present, Laubiericoncha is best considered as a monotypic genus with the only species L. myriamae distributed in the West Atlantic near off Barbados and in the Gulf of Mexico (1170–3234 m).
Discussion
The Pliocardiinae are found world-wide, primarily from cold seep environments, but also from hydrothermal vents and other sulphide-rich habitats that support their chemosymbiotic microbes (Johnson et al. 2017). The Southern Ocean populations of A. s.l. puertodeseadoi reported herein is the first record of live pliocardiines in subantarctic waters that definitively extends the range of the subfamily to the Southern Ocean.
Described, living taxa in Archivesica s.l. most closely related to A. s.l. puertodeseadoi on the basis of morphology and the topology of recent phylogenetic reconstructions (Johnson et al. 2017) include A. chuni, Archivesica angulata, Archivesica suavis, Archivesica laubieri, Archivesica laubieri kurilensis, Archivesica fortunata, and Archivesica nanshaensis. Of these species, A. angulata, A. suavis and A. fortunata are morphologically similar and future molecular evidence may show them to be synonyms. The distribution range of this group of pliocardiines is global and include East Pacific (A. suavis, A. angulata), West Pacific (A. nanshaensis, A. fortunata, A. laubieri, A. laubieri kurilensis), Atlantic (A. chuni), South Atlantic, and Southern Ocean (A. s.l. puertodeseadoi) (Krylova and Sahling 2010; Signorelli and Pastorino 2015; Johnson et al. 2017; Chen et al. 2018).
There are also fossil records of pliocardiines resembling A. s.l. puertodeseadoi. One was described from Svalbard (16 kyr, at 78°N, 1200 m depth) as Archivesica arctica Hansen et al. 2017, and another was reported from the Rainbow vent field (25.5 kyr, approximately 2.5 km east of Mid-Atlantic Ridge, 36°13′N, 1980 m depth) on the Mid-Atlantic Ridge and tentatively referred to as Phreagena s.l. sp. (Lartaud et al. 2010). Specimens from the Rainbow vent field are morphologically very close to A. s.l. puertodeseadoi and may actually be conspecific, indicating a possible wide distribution of this lineage in hydrothermal vents along the Mid-Atlantic Ridge in the Pleistocene. This would suggest migrations of the lineage between more northern Atlantic vent habitats and vents and seeps in the South Atlantic and Southern Ocean.
These systematic issues, and the potential for broad dispersal and ecological plasticity, are also relevant to interpreting the ecophysiology of pliocardiine species. Experimental physiology studies have been conducted on several species in the group, in multiple oceans, with differing results. The metabolic rates that we recorded are broadly in line with expectations from similar species, including the epilithic Turneroconcha magnifica (formerly called Calyptogena magnifica) with rates approximately 1.6–6.3 (MO2: μmol O2 g−1 h−1, calculated using dry mass) (Arp et al. 1984; Khripounoff et al. 2017; Krylova and Sahling 2020), and two other smaller species of Calyptogena had lower rates of MO2 (Childress and Mickel 1985).
Recent work using in situ measurements of oxygen consumption by pliocardiine clam assemblages have found widely separated rates even using the same species and the same equipment, including MO2 rate estimates for two other pliocardiine species of 16–26 (μmol O2 g−1 h−1, calculated with dry mass), considerably higher than previous experimental results (Khripounoff et al. 2017). The advantage of in situ respirometry, compared to the ex situ shipboard experiments used here, is that animals are not subjected to the stress of capture and consequent changes in temperature and pressure. The disadvantage is that in situ experiments of clams in sediment include additional respiration of other sediment organisms and biological processes, and there may be substantial group effects impacting total respiration even among the target species (e.g. Paterson 1983). Experiments on single individuals held in consistent conditions, such as in the present study, allow comparisons of the effects of discrete factors including body size and temperature.
The Metabolic Level Boundaries hypothesis predicts that polar marine animals may typically have very high metabolic scaling exponents, meaning that metabolic rate increases rapidly with increasing body size (Glazier 2010; Verberk and Atkinson 2013). Size-dependent variation in rates, coupled with the high variability seen in our experiments, would explain the deviations in rates recorded in multiple in situ experiments on other pliocardiine species. Metabolic rates that we observed in larger animals were highly variable, the scaling exponent could not be determined with confidence for these data; however, among the smallest individuals VO2 was substantially lower in those in the elevated temperature group. This is evidence for potential metabolic suppression at elevated temperatures in A. s.l. puertodeseadoi, which is a typical response to environmental stress in temperate cold-water molluscs (e.g. Carey and Sigwart 2014; Carey et al. 2016) and one other vent obligate mollusc (Sigwart and Chen 2018).
Thermal stress in the vent holobiont snail Alviniconcha marisindica prompted apparent symbiont bleaching, where material resembling bacterial mats was shed from the mantle cavity (Sigwart and Chen 2018); this was not observed in A. s.l. puertodeseadoi. Alviniconcha hosts symbionts in a network of vacuoles within its cells that open to the mantle cavity (Endow and Ohta 1989), whereas A. s.l. puertodeseadoi has true intracellular symbionts in its gill tissue and likely uses vertical trans-generational transfer of the symbiotic bacteria on the eggs, as reported for the closely related pliocardiine Phreagena okutanii (Ikuta et al. 2016). It is not wholly clear how chemosymbiosis may influence oxygen consumption compared to heterotrophic molluscs, and chemosymbiosis is prerequisite for these animals to occupy their niche.
Prior to examining experimental responses of metabolism in controlled conditions, our initial observations of the ecology of A. s.l. puertodeseadoi led us to speculate that the animals may be highly sensitive to temperature. The animals are seen living both buried within the sediment, which had substantial temperature elevation above ambient seawater, and epilithically where elevated temperatures could not be maintained. We would have expected that increases in sediment temperatures would prompt the bivalves to escape elevated temperatures by crawling to hard substrata (see video in Online Resource 1), as long as appropriate chemical conditions were available to support their symbiotic bacteria. Although experiments measuring oxygen consumption in response to temperature have certain limitations in understanding whole-animal physiology, the high variance in metabolic rates, and the lack of any clear change in VO2 or MO2 in our different temperature treatments, indicates that this species may be more tolerant to thermal fluctuations than expected.
Pliocardiines live at cold temperatures at the seabed-water interface (Arp et al. 1984; Decker et al. 2017). The bivalve’s siphons extended into the cold water for respiration, but the foot extends into warmer, anoxic, sulphide- or hydrocarbon-rich sediments to take up inorganic compounds such as hydrogen sulphide to supply endosymbiont microbes (e.g. Childress and Mickel 1985; Niemann et al. 2009). Most pliocardiines occur in patches, from one to several square metres in suitable sediments (e.g. Sahling et al. 2008). The single, long-dead specimen of vesicomyid collected from Ross Sea has a very large (28 cm shell length) and elongate shell and is clearly not conspecific with A. s.l. puertodeseadoi (Marshall and Tracey 2015), but it has not been sighted in its natural assemblage. Assemblages in Larsen A and Larsen B were around one to several metres wide (Domack et al. 2005; Niemann et al. 2009), which is within the normal range of pliocardiine patches (Johnson et al. 2017). By this standard the observations in Kemp Caldera are particularly widespread, with extensive coverage of live clams in patches with diameters from 5–10 m or even larger. The Kemp Caldera is also characterised by a widespread seafloor temperature anomaly which may be critical to support A. s.l. puertodeseadoi as well as other species that are associated but not obligate to hydrothermal activity.
Our observations indicate that pliocardiines, are able to adapt to living in a variety of habitats and biotopes, both epifaunally and infaunally (Fig. 5). Conditions appropriate to maintain symbiotic microbes are available in sulphur from active hydrothermal sites (Fig. 5a,b), like the Kemp Caldera (Cole et al. 2014; Hawkes et al. 2014), or from methane trapped under extended Antarctic ice shelves (Fig. 5c). Under these extended, hundreds of meters thick ice shelves with a restricted water column, geothermally originating methane seepage may provide sufficiently high density to support specialist communities. This idea was originally proposed by Domack et al. (2005) after the collapse of Larsen B in 2002. The 2005 discovery of dead pliocardiine assemblages in an area previously covered by the ice shelf, and the idea of under-ice-shelf seepage was further discussed by Ingels et al. (2018). The methane source for the Larsen B seep is thermogenic (and possibly hydrothermal), based on the hydrocarbon composition (Niemann et al. 2009), and recent evidence from the western Antarctic Peninsula (Larsen A-E areas) also showed positive geothermal anomalies (Jordan et al. 2017; Martos et al. 2017). The sediment conditions in a patch of dead A. s.l. puertodeseadoi in the Larsen B area led Niemann et al. (2009) to propose that methanotrophic bacteria related to ANME-3 (anaerobic methanotrophic archaea) most likely enable anaerobic methane oxidation in the Larsen B seep when the ice shelf was still present. Pliocardiine metabolism is indirectly based on anaerobic methane oxidation, which produces sulphide that in turn can be used as an energy source by the sulphur-oxidising symbionts that provide nutrition to the bivalve host (Boetius et al. 2000). The collapse or calving of extended ice shelves, like the recent break-up of the gigantic iceberg A68 from Larsen C (Hogg and Gudmundsson 2017), could release previously trapped geothermally originating methane seepage through the water column, remove the food source for the chemoautotrophic bacteria and lead to the local extinction of pliocardiine assemblages.
Schematic showing types of known habitats and assemblages of Archivesica s.l. puertodeseadoi in the Southern Ocean: a sulphidic sediments in hydrothermally active areas, b near sulphidic diffuse flow vents, c under ice shelf among bacterial mats, and d dead assemblages under calved ice shelf. The key at bottom indicates the major organism types found co-occuring with pliocardiine clams in these habitats, including actinostolid anemones, seastars in the genus Paulasterias, limpets in the genera Cocculinia and Lepetodrilus, pyconogonids in the genus Sericosura, and bacterial mats as well as geological features; not all organisms or features are present in all habitats
The nature of Antarctic habitats, and the dynamics of ice shelf growth and destruction may have prompted a new type of ephemeral habitat with geothermal methane trapped under ice, colonised by a single, ecologically flexible pliocardiine species. Our experimental evidence supports an inference of thermal tolerance. The morphological similarity of this species with pliocardiines broadly distributed through the deep Atlantic, including fossils, indicates potentially long-term dispersion over a large geographic area, despite the specialist habitat of this species. This follows a longer-term pattern in this diverse symbiotrophic bivalve radiation, which contains lineages adapted to organic falls, seeps, and vents. In the Antarctic, this pliocardiine bivalve can find its habitat and achieve large biomasses equally in sulphidic sediments, diffuse geothermal flow, or methane seepage amplified in under-ice water masses.
Dedication
This publication is dedicated to our colleague and friend Heiko Sahling, who passed away on 23 April 2018. He initiated the collaboration between Katrin Linse and Elena Krylova on this topic and hosted them in his office at the initial stage of this study. The area of Heiko’s scientific activity included both marine geochemistry and biology, and his deep understanding of geochemical processes together with his biological knowledge resulted in studies that have become great contribution to research of deep-sea oceanology.
References
Allen JA (2001) The family Kelliellidae (Bivalvia: Heterodonta) from the deep Atlantic and its relationship with the family Vesicomyidae. Zool J Linn Soc 131:199–226. https://doi.org/10.1006/zjls.2000.0233
Arango CP, Linse K (2015) New Sericosura (Pycnogonida: Ammotheidae) from deep-sea hydrothermal vents in the Southern Ocean. Zootaxa 3995:037–050. https://doi.org/10.11646/zootaxa.3995.1.5
Arp AJ, Childress JJ, Fisher CRJ (1984) Metabolic and blood gas transport characteristics of the hydrothermal vent bivalve Calyptogena magnifica. Physiol Zool 57:648–662. https://doi.org/10.1086/physzool.57.6.30155991
Audzijonyte A, Krylova EM, Sahling H, Vrijenhoek RC (2012) Molecular taxonomy reveals broad trans-oceanic distributions and high species diversity of deep-sea clams (Bivalvia: Vesicomyidae: Pliocardiinae) in chemosynthetic environments. Syst Biodivers 10:403–415. https://doi.org/10.1080/14772000.2012.744112
Baco AR, Smith CR, Roderick GK, Peek AS, Vrijenhoek RC (1999) The phylogenetic relationships of whale-fall vesicomyid clams based on mitochondrial COI DNA sequences. Mar Ecol Prog Ser 182:137–147. https://doi.org/10.3354/meps182137
Benson BB, Krause D Jr (1984) The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol Oceanogr 29:620–632. https://doi.org/10.4319/lo.1984.29.3.0620
Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, Amann R, Jørgensen BB, Witte U, Pfannkuche O (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623–626. https://doi.org/10.1038/35036572
Carey N, Dupont S, Sigwart JD (2016) Sea hare Aplysia punctata (Mollusca: Gastropoda) can maintain shell calcification under extreme ocean acidification. The Biological Bulletin 231:142–151. https://doi.org/10.1086/690094
Carey N, Sigwart JD (2014) Size matters: plasticity in metabolic scaling shows body-size may modulate responses to climate change. Biol Lett 10:20140408. https://doi.org/10.1098/rsbl.2014.0408
Carey N, Sigwart JD, Richards JG (2013) Economies of scaling: More evidence that allometry of metabolism is linked to activity, metabolic rate and habitat. J Exp Mar Biol Ecol 439:7–14. https://doi.org/10.1016/j.jembe.2012.10.013
Chen C, Okutani T, Liang Q, Qiu J-W (2018) A noteworthy new species of the family Vesicomyidae from the South China Sea (Bivalvia: Glossoidea). Venus 76:29–37
Chen C, Linse K (2020) From wood to vent: first cocculinid limpet associated with hydrothermal activity discovered in the Southern Ocean. Antarct Sci. https://doi.org/10.1017/S09541202000022X
Childress JJ, Mickel TJ (1985) Metabolic rates of animals from the hydrothermal vents and other deep-sea habitats. Bull Biol Soc Washington 6:249–260
Cole CS, James RH, Connelly DP, Hathorne EC (2014) Rare earth elements as indicators of hydrothermal processes within the East Scotia subduction zone system. Geochim Cosmochim Ac 140:20–38. https://doi.org/10.1016/j.gca.2014.05.018
Corliss JB, Dymond J, Gordon LI, Edmond JM, von Herzen RP, Ballard RD, Green K, Williams D, Bainbridge A, Crane K, van Andel TH (1979) Submarine thermal springs on the Galápagos Rift. Science 203:1073–1083. https://doi.org/10.1126/science.203.4385.1073
Cosel Rv, Salas C (2001) Vesicomyidae (Mollusca: Bivalvia) of the genera Vesicomya, Waisiuconcha, Isorropodon and Callogonia in the eastern Atlantic and the Mediterranean. Sarsia 86:333–366
Cosel Rv, Olu K (2008) A new genus and new species of Vesicomyidae (Mollusca, Bivalvia) from cold seeps on the Barbados accretionary prism, with comments on other species. Zoosystema 30:929–944
Cosel Rv, Olu K (2009) Large Vesicomyidae (Mollusca: Bivalvia) from cold seeps in the Gulf of Guinea off the coasts of Gabon, Congo and northern Angola. Deep-Sea Res Pt II 56:2350–2379. https://doi.org/10.1016/j.dsr2.2009.04.016
Decker C, Olu K, Cunha RL, Arnaud-Haond S (2012) Phylogeny and diversification patterns among vesicomyid bivalves. PLoS ONE 7:e33359. https://doi.org/10.1371/journal.pone.0033359
Decker C, Zorn N, Le Bruchec J, Caprais JC, Potier N, LallierFH L-W, Olu K, Andersen AC (2017) Can the hemoglobin characteristics of vesicomyid clam species influence their distribution in deep-sea sulfide-rich sediments? a case study in the Angola Basin. Deep-Sea Res Part II 142:219–232. https://doi.org/10.1016/j.dsr2.2016.11.009
Domack E, Ishman S, Leventer A, Sylva S, Willmott V, Huber BE (2005) A chemotrophic ecosystem found beneath Antarctic ice shelf. EOS Trans Am Geophys Union 86:269–276
Dubilier N, Bergin C, Lott C (2008) Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat Rev Microbiol 6:725–740. https://doi.org/10.1038/nrmicro1992
Egorova EN (1998) Two new species of deep-water bivalve from the Weddell Sea, West Antarctica (Mollusca: Kelliellidae and Cuspidaridae). Zoosystematica Rossica 7:245–249
Endow K, Ohta S (1989) The symbiotic relationship between bacteria and a mesogastropod snail, Alviniconcha hessleri, collected from hydrothermal vents of the Mariana Back-Arc Basin. Bull J Soc Microbial Ecol 3:73–82
Fisher CR, Childress JJ, Arp AJ, Brooks JM, Distel DL, Dugan JA, Felbeck H, Fritz LW, Hessler RR, Johnson KS, Kennicutt MC, Lutz RA, Macko SA, Newton A, Powell MA, Somero GN, Soto T (1988) Variation in the hydrothermal vent clam, Calyptogena magnifica, at the Rose Garden vent on the Galapagos spreading center. Deep-Sea Res Pt A 35:1811–1831. https://doi.org/10.1016/0198-0149(88)90051-9
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Giere O, Borowski C, Prieur D (2003) Chapter 10: Biological productivity in hydrothermal systems. In: Halbach PE, et al. (eds) Energy and mass transfer in marine hydrothermal systems. Dahlem University Press, Berlin, pp 111–233
Glazier DS (2010) A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev 85:111–138. https://doi.org/10.1111/j.1469-185X.2009.00095.x
Gutt J (2008) The Expedition ANTARKTIS-XXIII/8 of the Research Vessel "Polarstern" in 2006/2007. Reports on Polar and Marine Research 569:1–153
Hansen J, Hoff U, Sztybor K, Rasmussen TL (2017) Taxonomy and palaeoecology of two Late Pleistocene species of vesicomyid bivalves from cold methane seeps at Svalbard (79°N). J Molluscan Stud 83:270–279. https://doi.org/10.1093/mollus/eyx014
Hawkes JA, Connelly DP, Rijkenberg MJA, Achterberg EP (2014) The importance of shallow hydrothermal island arc systems in ocean biogeochemistry. Geophys Res Lett 41:942–947. https://doi.org/10.1002/2013GL058817
Hecker B (1985) Fauna from a cold sulfur-seep in the Gulf of Mexico: Comparison with hydrothermal vent communities and evolutionary implications. Bull Biol Soc Washington 6:465–473
Hogg AE, Gudmundsson GH (2017) Impacts of the Larsen-C Ice Shelf calving event. Nat Clim Change 7:540. https://doi.org/10.1038/nclimate3359
Ikuta T, Igawa K, Tame A, Kuroiwa T, Kuroiwa H, Aoki Y, Takaki Y, Nagai Y, Ozawa G, Yamamoto M, Deguchi R, Fujikura K, Maruyama T, Yoshida T (2016) Surfing the vegetal pole in a small population: extracellular vertical transmission of an 'intracellular' deep-sea clam symbiont. R Soc Open Sci 3:160130. https://doi.org/10.1098/rsos.160130
Ingels J, Aronson RB, Smith CR (2018) The scientific response to Antarctic ice-shelf loss. Nat Clim Change 8:848–851. https://doi.org/10.1038/s41558-018-0290-y
Johnson SB, Krylova EM, Audzijonyte A, Sahling H, Vrijenhoek RC (2017) Phylogeny and origins of chemosynthetic vesicomyid clams. Syst Biodivers 15:346–360. https://doi.org/10.1080/14772000.2016.1252438
Jordan TA, Ferraccioli F, Leat PT (2017) New geophysical compilations link crustal block motion to Jurassic extension and strike-slip faulting in the Weddell Sea Rift System of West Antarctica. Gondwana Res 42:29–48. https://doi.org/10.1016/j.gr.2016.09.009
Khripounoff A, Caprais JC, Decker C, Le Bruchec J, Noel P, Husson B (2017) Respiration of bivalves from three different deep-sea areas: Cold seeps, hydrothermal vents and organic carbon-rich sediments. Deep-Sea Res Pt II 142:233–243. https://doi.org/10.1016/j.dsr2.2016.05.023
Knust R (2012) The Expedition of the Research Vessel "Polarstern" to the Antarctic in 2011 (ANT-XXVII/83) (CAMBIO). Reports on Polar and Marine Research 644:1–205
Krylova EM, Kamenev GM, Vladychenskaya IP, Petrov NB (2015) Vesicomyinae (Bivalvia: Vesicomyidae) of the Kuril-Kamchatka Trench and adjacent abyssal regions. Deep-Sea Res Pt II 111:198–209. https://doi.org/10.1016/j.dsr2.2014.10.004
Krylova EM, Sahling H (2006) Recent bivalve molluscs of the genus Calyptogena (Vesicomyidae). J Molluscan Stud 72:359–395. https://doi.org/10.1093/mollus/eyl022
Krylova EM, Sahling H (2010) Vesicomyidae (Bivalvia): Current taxonomy and distribution. PLoS ONE 5:e9957. https://doi.org/10.1371/journal.pone.0009957
Krylova EM, Sahling H (2020) A new genus Turneroconcha (Bivalvia: Vesicomyidae: Pliocardiinae) for the giant hydrothermal vent clam ‘Calyptogena’ magnifica. Zootaxa 4808:79–100. https://doi.org/10.11646/zootaxa.4808.1.4
Krylova EM, Sahling H, Borowski C (2018) Resolving the status of the families Vesicomyidae and Kelliellidae (Bivalvia: Venerida), with notes on their ecology. J Molluscan Stud 84:69–91. https://doi.org/10.1093/mollus/eyx050
Krylova EM, Sellanes J, Valdés F, D’Elía G (2014) Austrogena: a new genus of chemosymbiotic bivalves (Bivalvia; Vesicomyidae; Pliocardiinae) from the oxygen minimum zone off central Chile described through morphological and molecular analyses. Syst Biodivers 12:225–246. https://doi.org/10.1080/14772000.2014.900133
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lartaud F, de Rafelis M, Oliver G, Krylova E, Dyment J, Ildefonse B, Thibaud R, Gente P, Hoisé E, Meistertzheim A-L, Fouquet Y, Gaill F, Le Bris N (2010) Fossil clams from a serpentinite-hosted sedimented vent field near the active smoker complex Rainbow, MAR, 36°13′N: Insight into the biogeography of vent fauna. Geochem Geophys Geosy. https://doi.org/10.1029/2010GC003079
Leat PT, Day SJ, Tate AJ, Martin TJ, Owen MJ, Tappin DR (2013) Volcanic evolution of the South Sandwich volcanic arc, South Atlantic, from multibeam bathymetry. J Volcanol Geoth Res 265:60–77. https://doi.org/10.1016/j.jvolgeores.2013.08.013
Leat PT, Fretwell PT, Tate AJ, Larter RD, Martin TJ, Smellie JL, Jokat W, Bohrmann G (2016) Bathymetry and geological setting of the South Sandwich Islands volcanic arc. Antarct Sci 28:293–303. https://doi.org/10.1017/S0954102016000043
Levin LA (2005) Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanogr Mar Biol 43:1–46. https://doi.org/10.1201/9781420037449.ch1
Linse K (2004) Scotia Arc deep-water bivalves: composition, distribution and relationship to the Antarctic shelf fauna. Deep-Sea Res Pt II 51:1827–1837. https://doi.org/10.1016/j.dsr2.2004.07.016
Linse K (2014) Chapter 5.11. Bivalves. In: De Broyer C, et al. (eds) The Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 126–128
Linse K, Copley JT, Connelly DP, Larter RD, Pearce DA, Polunin NV, Rogers AD, Chen C, Clarke A, Glover AG, Graham AG (2019a) Fauna of the Kemp Caldera and its upper bathyal hydrothermal vents (South Sandwich Arc, Antarctica). Roy Soc Open Sci 6:191501
Linse K, Rotermann CN, Chen C (2019b) A new vent limpet in the genus Lepetodrilus (Gastropoda: Lepetodrilidae) from Southern Ocean hydrothermal vent fields showing high phenotypic plasticity. Front Marine Sci. https://doi.org/10.3389/fmars.2019.00381
Littlewood DTJ (1994) Molecular Phylogenetics of Cupped Oysters Based on Partial 28S rRNA Gene Sequences. Mol Phylogen Evol 3:221–229. https://doi.org/10.1006/mpev.1994.1024
Mah C, Linse K, Copley JT, Marsh L, Rogers A, Clague D, Foltz D (2015) Description of a new family, including new genera and species of deep-sea Forcipulatacea (Asteroidea), the first known sea star from hydrothermal vent settings. Zool J Linn Soc 174:93–113
Marsh L, Copley JT, Huvenne VAI, Tyler PA, The Isis ROV Facility (2013) Getting the bigger picture: Using precision Remotely Operated Vehicle (ROV) videography to acquire high-definition mosaic images of newly discovered hydrothermal vents in the Southern Ocean. Deep-Sea Res Pt II 92:124–135. https://doi.org/10.1016/j.dsr2.2013.02.007
Marshall B, Tracey D (2015) First evidence for deep-sea hot venting or cold seepage in the Ross Sea (Bivalvia: Vesicomyidae). The Nautilus 129:140–141
Martos YM, Catalán M, Jordan TA, Golynsky A, Golynsky D, Eagles G, Vaughan DG (2017) Heat flux distribution of Antarctica unveiled. Geophys Res Lett 44:11417–11426. https://doi.org/10.1002/2017GL075609
Medlin L, Elwood HJ, Stickel S, Sogin ML (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491–499. https://doi.org/10.1016/0378-1119(88)90066-2
Niemann H, Fischer D, Graffe D, Knittel K, Montiel A, Heilmayer O, Nöthen K, Pape T, Kasten S, Bohrmann G, Boetius A, Gutt J (2009) Biogeochemistry of a low-activity cold seep in the Larsen B area, western Weddell Sea, Antarctica. Biogeosciences 6:2383–2395. https://doi.org/10.5194/bg-6-2383-2009
Paterson CG (1983) Effect of aggregation on the respiration rate of the freshwater unionid bivalve, Elliptio complanata (Solander). Freshwater Invertebrate Biology 2:139–146. https://doi.org/10.2307/1467088
Paull CK, Hecker B, Commeau R, Freeman-Lynde RP, Neumann C, Corso WP, Golubic S, Hook JE, Sikes E, Curray J (1984) Biological communities at the Florida Escarpment resemble hydrothermal vent taxa. Science 226:965–967. https://doi.org/10.1126/science.226.4677.965
Pelseneer P (1903) Mollusques (amphineures, gastropodes et lamellibranches), Resultats du Voyage du S.Y. Belgica en 1897–1898–1899 Rapports Scientifiques, Zoologie. Buschmann, Antwerpen.
Rogers AD (2010) Chemosynthetic Ecosystems of the Southern Ocean (CHESSO): RRS James Cook Cruise 42. RRS James Cook Cruise Report 42:1–240. https://www.bodc.ac.uk/resources/inventories/cruise_inventory/reports/jc042.pdf
Rogers AD, Tyler PA, Connelly DP, Copley JT, James R, Larter RD, Linse K, Mills RA, Garabato AN, Pancost RD, Pearce DA, Polunin NVC, German CR, Shank T, Boersch-Supan PH, Alker BJ, Aquilina A, Bennett SA, Clarke A, Dinley RJJ, Graham AGC, Green DRH, Hawkes JA, Hepburn L, Hilario A, Huvenne VAI, Marsh L, Ramirez-Llodra E, Reid WDK, Roterman CN, Sweeting CJ, Thatje S, Zwirglmaier K (2012) The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol 10:e1001234. https://doi.org/10.1371/journal.pbio.1001234
Roterman CN, Copley JT, Linse KT, Tyler PA, Rogers AD (2016) Connectivity in the cold: the comparative population genetics of vent-endemic fauna in the Scotia Sea, Southern Ocean. Mol Ecol 25:1073–1088. https://doi.org/10.1111/mec.13541
Sahling H, Bohrmann G, Spiess V, Bialas J, Breitzke M, Ivanov M, Kasten S, Krastel S, Schneider R (2008) Pockmarks in the Northern Congo Fan area, SW Africa: Complex seafloor features shaped by fluid flow. Mar Geol 249:206–225. https://doi.org/10.1016/j.margeo.2007.11.010
Signorelli J, Pastorinio G (2015) A new species of Laubiericoncha (Bivalvia: Vesicomyidae) from deep waters off Argentina. Malacologia 58:349–360
Sigwart JD, Chen C (2018) Comparative oxygen consumption of gastropod holobionts from deep-sea hydrothermal vents in the Indian Ocean. Biol Bull 235:102–112. https://doi.org/10.1086/699326
Taylor J, Glover E (2010) Chemosymbiotic bivalves. In: Kiel S (ed) Topics in Geobiology 33: The vent and seep biota. Springer, The Netherlands, pp 107–135
Thiele J, Jaeckel S (1931) Muscheln der deutschen Tiefsee-Expedition Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer. Valdivia 1898–1899(21):160–268
Verberk WCEP, Atkinson D (2013) Why polar gigantism and Palaeozoic gigantism are not equivalent: effects of oxygen and temperature on the body size of ectotherms. Funct Ecol 27:1275–1285. https://doi.org/10.1111/1365-2435.12152
Vermeij GJ (2016) Gigantism and Its Implications for the History of Life. PLoS ONE 11:e0146092. https://doi.org/10.1371/journal.pone.0146092
Acknowledgements
We thank the scientific cruise leaders Prof Alex Rogers and Prof Gerhard Bohrmann, the masters and crews of RRS James Clark Ross, RRS James Cook and RV Polarstern, and science teams onboard for logistic, technical and shipboard support during JC42. We especially acknowledge the ROV teams from NMF and MARUM for their dedication to collect our samples with ROVs Isis and MARUM QUEST. We are grateful to NERC for funding the ChEsSo Consortium Grant (NE/DO1249X/1) under the lead of Prof Paul Tyler, and to BMBF and MARUM for funding PS119 and MARUM QUEST via grants to Prof Gerhard Bohrmann. We thank Robert S. Carney, Eric E. Cordes, Virginie Héros and Rudo von Cosel (MNHN), Tina Molodtsova (IORAN) for providing specimens of L. myriamae and A. s.l. chuni for this study. We thank Bruce Marshall, James McClintock, and one other anonymous reviewer for comments that improved an earlier version of this paper.
Funding
The ChEsSo research programme was funded by a NERC Consortium Grant (NE/DO1249X/1) and supported by the Census of Marine Life and the Sloan Foundation all of which are gratefully acknowledged. PS119 was funded by BMBF and MARUM. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. E. Krylova was supported by State assignment of Minobrnauki, Russia, Theme No. 0149–2019-0009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they do not have any financial, personal, or professional interests that could be construed to have influenced their paper.
Ethical approval
All necessary permits were obtained for the described field studies. Studies in the East Scotia Sea were undertaken under the permit S3-3/2009 issued by the Foreign and Commonwealth Office, London to Sect. 3 of the Antarctic Act 1994 and permit RAP 2018/064 (PS119) issued by the South Georgia and South Sandwich Government.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1. Time lapse video of live clams on board RV Polarstern held at ~4 °C. Video (1:21) represents approximately 10 hours in time lapse, taken on 12 May 2018 of specimens collected by the ROV MARUM-QUEST (dive 448) on 11 May 2018 in Kemp Caldera, 1427 m depth. Video courtesy of Yi-Ting Tseng, Marum, Bremen, Germany. Supplementary file1 (MP4 49704 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Linse, K., Sigwart, J.D., Chen, C. et al. Ecophysiology and ecological limits of symbiotrophic vesicomyid bivalves (Pliocardiinae) in the Southern Ocean. Polar Biol 43, 1423–1437 (2020). https://doi.org/10.1007/s00300-020-02717-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02717-z