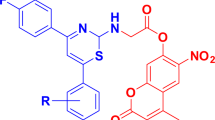
An array of previously synthesized 4-methyl-7-amino and amido coumarins 4a–u has been screened for their antimicrobial and antioxidant properties. Some of the compounds exhibited promising antibacterial and antifungal activities (MIC ranging from 4–64 μg/mL) when compared to the respective standards. Compound 4u showed comparable antibacterial activity with the standard, ciprofloxacin, whereas compounds 4u and 4t displayed promising antifungal activity when compared to the standard, fluconazole. The in silico docking studies against gyrase enzyme revealed the fact that 4u possessed hydrogen bonding and significant hydrophobic interactions, which may be the reason for its superior antibacterial activity as compared to the other compounds. Compounds 4c and 4m showed comparable antioxidant activity with the standard, BHT, which can be attributed to the presence of electron-donating substituents.


Similar content being viewed by others
References
F. Borges, F. Roleira, N. Milhazes, L. Santana, and E. Uriarte, Curr. Med. Chem., 12, 887 (2005).
M. A. Al-Haiza and M. S. Mostafa, Molecules, 8, 275 (2003).
K. C. Fylaktakidou and D. H. Litina, J. Curr. Pharm. Des., 10, 3813 (2004).
N. Lall, A. A. Hussein, and J. J. M. Meyer, Fitoterapia, 77, 230 (2006).
I. Kostova, S. Raleva, P. Genova, and R. Argirova, Bioinorg. Chem. Appl., 2006, 1 (2006).
J. W. Hinman, H. Hoeksema, E. L. Caron, and W. G. Jackson, J. Am. Chem. Soc., 78, 1072 (1956).
Shaveta, S. Mishra, and P. Singh, Eur. J. Med. Chem., 124, 500 (2016).
K. Hemalatha and G. Madhumitha, Eur. J. Med. Chem., 123, 596 (2016).
M. N. Joy, Y. D. Bodke, K. K. A. Khader, M. S. A. Padusha, A. M. Sajith, and A. Muralidharan, RSC Adv., 4, 19766 (2014).
A. M. Sajith, K. K. A. Khader, N. Joshi, M. N. Reddy, M. S. A. Padusha, H. P. Nagaswarupa, M. N. Joy, Y. D. Bodke, R. P. Karuvalam, R. Banerjee, A. Muralidharan, and P. Rajendra, Eur. J. Med. Chem., 89, 21 (2015).
C. Aswathanarayanappa, E. Bheemappa, Y. D. Bodke, P. S. Krishnegowda, S. P. Venkata, and R. Ningegowda, Arch. Pharm. Chem. Life Sci., 346, 922 (2013).
M. K. Potdar, S. S. Mohile, and M. M. Salunkhe, Tetrahedron Lett., 42, 9285 (2001).
M. A. Parker, D. M. Kurrasch, and D. E. Nichols, Bioorg. Med. Chem., 16, 4661 (2008).
E. Niki, Chem. Phys. Lipids, 44, 227 (1987).
M. J. Matos, F. P. Cruz, S. V. Rodriguez, E. Uriarte, L. Santana, F. Borges, and C. O. Azar, Bioorg. Med. Chem., 21, 3900 (2013).
C. Yamagami, M. Akamatsu, N. Motohashi, S. Hamada, and T. Tanahashi, Bioorg. Med. Chem. Lett., 15, 2845 (2005).
B. J. Bradbury and M. J. Pucci, Curr. Opin. Pharmacol., 8, 574 (2008).
Y. C. Tse-Dinh, Infect. Disord. Drug Targets, 7, 3 (2007).
B. A. A. Skaggs, M. Molestely, D. W. Warnock, and C. J. Morrison, J. Clin. Microbiol., 38, 2254 (2000).
D. J. M. Lowry, M. J. Jaqua, and S. T. Selepak, Appl. Microbiol., 20, 46 (1970).
A. Braca, N. D. Tommasi, L. D. Bari, C. Pizza, M. Politi, and I. Morelli, J. Nat. Prod., 64, 892 (2001).
T. Sander, J. Freyss, M. V. Korff, J. R. Reich, and C. Rufener, J. Chem. Inform. Model., 49, 232 (2009).
O. Trott and A. J. Olson, J. Comput. Chem., 31, 455 (2010).
B. D. Bax, P. F. Chan, D. S. Eggleston, A. Fosberry, D. R. Gentry, and F. Gorrec, Nature, 466, 935 (2010).
G. M. Morris, D. S. Goodsell, R. S. Halliday, R. Huey, W. E. Hart, and R. K. Belew, J. Comput. Chem., 19, 1639 (1998).
R. A. Laskowski and M. B. Swindells, J. Chem. Inform. Model., 51, 2778 (2011).
W. DeLano, The PyMOL Molecular Graphics System (2002), http://www.citeulike.org/group/340/article/240061 ().
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2020, pp. 531–536.
Rights and permissions
About this article
Cite this article
Joy, M.N., Bodke, Y.D. & Telkar, S. 4-Methyl-7-Amino/Amido Coumarin Derivatives as Potential Antimicrobials and Antioxidants. Chem Nat Compd 56, 614–620 (2020). https://doi.org/10.1007/s10600-020-03106-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03106-y