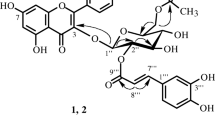
New flavonoids were isolated from the aerial part of Artemisia frigida Willd. (Asteraceae). Their structures were elucidated using UV, IR, and NMR spectroscopy and mass spectrometry as chrysoeriol-7-O-(2′′-Oacetyl)-β-D-glucopyranoside (2′′-O-acetylthermopsoside), jaceosidin-4′ -O-β-D-glucopyranoside (isojaceoside), and 13 known flavonoids. The influence of the isolated compounds on α-glucosidase activity and triglyceride accumulation in 3T3-L1 preadipocytes was studied.

Similar content being viewed by others
References
R. X. Tan, W. F. Zheng, and H. Q. Tang, Planta Med., 64, 295 (1998).
K. S. Bora and A. Sharma, Pharm. Biol., 49, 101 (2011).
D. N. Olennikov, N. K. Chirikova, N. I. Kashchenko, V. M. Nikolaev, S.-W. Kim, and C. Vennos, Front. Pharmacol., 9, 756 (2018).
D. N. Olennikov, N. I. Kashchenko, N. K. Chirikova, A. G. Vasil’eva, A. I. Gadimli, J. I. Isaev, and C. Vennos, Antioxidants, 8, 307 (2019).
K. I. Alipieva, E. P. Kostadinova, L. N. Evstatieva, M. Stefova, and V. S. Bankova, Fitoterapia, 80, 51 (2009).
T. Iwashina, K. Kamenosono, and T. Ueno, Phytochemistry, 51, 1109 (1999).
D. N. Olennikov and N. I. Kashchenko, Chem. Nat. Compd., 55, 256 (2019).
D. N. Olennikov, N. I. Kashchenko, N. K. Chirikova, and L. M. Tankhaeva, Molecules, 20, 19172 (2015).
M. A. M. Nawwar, S. A. M. Hussein, and I. Merfort, Phytochemistry, 37, 1175 (1994).
H. Wagner, L. Horhammer, R. Hoer, T. Murakami, and L. Farkas, Tetrahedron Lett., 10, 3411 (1969).
S. Kh. Faizieva, Z. A. Khusbaktova, V. N. Syrov, M. P. Yuldashev, E. Kh. Batirov, and Sh. Sh. Sagdullaev, Chem. Nat. Compd., 35, 155 (1999).
S. A. Basudan, M. Ilyas, M. Parveen, H. M. Muhisen, and R. Kumar, J. Asian Nat. Prod. Res., 7, 81 (2005).
H. R. Monsef-Esfahani, R. Hajiaghaee, A. R. Shahverdi, M. R. Khorramizadeh, and M. Amini, Pharm. Biol., 48, 333 (2010).
N. K. Chirikova, D. N. Olennikov, and L. M. Tankhaeva, Russ. J. Bioorg. Chem., 36, 915 (2010).
D. de Beer, E. Joubert, C. J. Malherbe, and J. D. Brand, J. Chromatogr. A, 1218, 6179 (2011).
K. R. Markham, Techniques of Flavonoid Identification, Academic Press, London, New York, 1982, 113 pp.
A. Lenherr, M. F. Lahloub, and O. Sticher, Phytochemistry, 23, 2343 (1984).
D. N. Olennikov, A. I. Gadimli, J. I. Isaev, N. I. Kashchenko, A. S. Prokopyev, T. N. Katayeva, N. K. Chirikova, and C. Vennos, Metabolites, 9, 271 (2019).
P. Bashyal, P. Parajuli, R. P. Pandey, and J. K. Sohng, Catalysts, 9, 112 (2019).
G. She, Z. Guo, H. Lv, and D. She, Molecules, 14, 4190 (2009).
K. R. Markham, B. Ternai, R. Stanley, H. Geiger, and T. J. Mabry, Tetrahedron, 34, 1389 (1978).
B. Ali, M. Imran, R. Hussain, Z. Ahmed, and A. Malik, Magn. Reson. Chem., 48, 159 (2010).
Y. Lu, Y. Sun, L. Y. Foo, W. C. McNabb, and A. L. Molan, Phytochemistry, 55, 67 (2000).
Z. Hajdu, A. Martins, O. Orban-Gyapai, P. Forgo, N. Jedlinszki, I. Mathe, and J. Hohmann, Rec. Nat. Prod., 8, 299 (2014).
D. N. Olennikov, N. K. Chirikova, N. I. Kashchenko, T. G. Gornostai, I. Y. Selyutina, and I. N. Zilfikarov, Int. J. Mol. Sci., 18, 2579 (2017).
M. Akabane, A. Yamamoto, S. Aizawa, A. Taga, and S. Kodama, Anal. Sci., 30, 739 (2014).
N. I. Kashchenko, N. K. Chirikova, and D. N. Olennikov, Molecules, 22, 73 (2017).
D. N. Olennikov, I. A. Fedorov, N. I. Kashchenko, N. K. Chirikova, and C. Vennos, Molecules, 24, 2286 (2019).
ACKNOWLEDGMENT
The studies were performed with support from FASO Russia (AAAA-A17-117011810037-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2020, pp. 539–543.
Rights and permissions
About this article
Cite this article
Olennikov, D.N. New Flavonoids from Artemisia frigida. Chem Nat Compd 56, 623–627 (2020). https://doi.org/10.1007/s10600-020-03108-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03108-w