Abstract
Background
Mitoxantrone (MTX) is a synthetic compound used as a second line chemotherapeutic drug for prostate cancer. It has been reported to trigger immunogenic cell death (ICD) in animal model studies, but the underlying mechanism is not fully understood yet, especially not in prostate cancer cells.
Methods
ICD was determined by assessing the release of damage-associated molecular patterns (DAMPs) in the prostate cancer-derived cell lines LNCaP, 22RV1 and PC-3. Short hairpin RNAs (shRNAs) were used to knock down target gene expression. Phagocytosis was assessed using a dual labeling technology in dendric cells co-cultured with cancer cells. The PERK gene promoter was cloned for dual luciferase assays. Chromatin immunoprecipitation (ChIP) was used to determine p53 protein-DNA binding activity. Immunocompetent mice and murine RM-1 prostate cancer cells were used for vaccination experiments.
Results
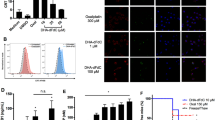
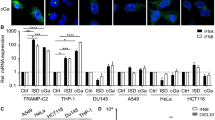
MTX treatment induced typical characteristics of DAMP release, including increased cell surface exposure of calreticulin (CALR), and extracellular release of ATP and high mobility group box-1 (HMGB1) protein. MTX also enhanced phagocytosis by dendritic cells. Moreover, MTX treatment increased eukaryotic initiation factor 2α (eIF2α) S51 phosphorylation, which was reduced when PERK and GCN2 were silenced using shRNAs. In addition, PERK or GCN2 silencing significantly reduced MTX-induced release of DAMPs in vitro and anti-tumor immunity in vivo. MTX treatment also resulted in dendritic cell activation in mice, which was attenuated when PERK or GCN2 were silenced in cancer cells used for vaccination. Further analysis revealed that PERK and GCN2 expression was enhanced by MTX treatment, of which PERK, but not GCN2, was enhanced via a p53-dependent mechanism.
Conclusion
MTX triggers ICD by activating eIF2α via PERK/GCN2 upregulation in prostate cancer cells. MTX-induced PERK expression upregulation depends on the p53 pathway, while that of GCN2 requires further investigation.









Similar content being viewed by others
References
L. Galluzzi, I. Vitale, S.A. Aaronson, J.M. Abrams, D. Adam, P. Agostinis, E.S. Alnemri, L. Altucci, I. Amelio, D.W. Andrews, M. Annicchiarico-Petruzzelli, A.V. Antonov, E. Arama, E.H. Baehrecke, N.A. Barlev, N.G. Bazan, F. Bernassola, M.J.M. Bertrand, K. Bianchi, M.V. Blagosklonny, K. Blomgren, C. Borner, P. Boya, C. Brenner, M. Campanella, E. Candi, D. Carmona-Gutierrez, F. Cecconi, F.K. Chan, N.S. Chandel, E.H. Cheng, J.E. Chipuk, J.A. Cidlowski, A. Ciechanover, G.M. Cohen, M. Conrad, J.R. Cubillos-Ruiz, P.E. Czabotar, V. D'Angiolella, T.M. Dawson, V.L. Dawson, V. De Laurenzi, R. De Maria, K.M. Debatin, R.J. DeBerardinis, M. Deshmukh, N. Di Daniele, F. Di Virgilio, V.M. Dixit, S.J. Dixon, C.S. Duckett, B.D. Dynlacht, W.S. El-Deiry, J.W. Elrod, G.M. Fimia, S. Fulda, A.J. Garcia-Saez, A.D. Garg, C. Garrido, E. Gavathiotis, P. Golstein, E. Gottlieb, D.R. Green, L.A. Greene, H. Gronemeyer, A. Gross, G. Hajnoczky, J.M. Hardwick, I.S. Harris, M.O. Hengartner, C. Hetz, H. Ichijo, M. Jaattela, B. Joseph, P.J. Jost, P.P. Juin, W.J. Kaiser, M. Karin, T. Kaufmann, O. Kepp, A. Kimchi, R.N. Kitsis, D.J. Klionsky, R.A. Knight, S. Kumar, S.W. Lee, J.J. Lemasters, B. Levine, A. Linkermann, S.A. Lipton, R.A. Lockshin, C. Lopez-Otin, S.W. Lowe, T. Luedde, E. Lugli, M. MacFarlane, F. Madeo, M. Malewicz, W. Malorni, G. Manic, J.C. Marine, S.J. Martin, J.C. Martinou, J.P. Medema, P. Mehlen, P. Meier, S. Melino, E.A. Miao, J.D. Molkentin, U.M. Moll, C. Munoz-Pinedo, S. Nagata, G. Nunez, A. Oberst, M. Oren, M. Overholtzer, M. Pagano, T. Panaretakis, M. Pasparakis, J.M. Penninger, D.M. Pereira, S. Pervaiz, M.E. Peter, M. Piacentini, P. Pinton, J.H.M. Prehn, H. Puthalakath, G.A. Rabinovich, M. Rehm, R. Rizzuto, C.M.P. Rodrigues, D.C. Rubinsztein, T. Rudel, K.M. Ryan, E. Sayan, L. Scorrano, F. Shao, Y. Shi, J. Silke, H.U. Simon, A. Sistigu, B.R. Stockwell, A. Strasser, G. Szabadkai, S.W.G. Tait, D. Tang, N. Tavernarakis, A. Thorburn, Y. Tsujimoto, B. Turk, T. Vanden Berghe, P. Vandenabeele, M.G.V. Heiden, A. Villunger, H.W. Virgin, K.H. Vousden, D. Vucic, E.F. Wagner, H. Walczak, D. Wallach, Y. Wang, J.A. Wells, W. Wood, J. Yuan, Z. Zakeri, B. Zhivotovsky, L. Zitvogel, G. Melino, G. Kroemer, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25, 486–541 (2018). https://doi.org/10.1038/s41418-017-0012-4
D.R. Green, The coming decade of cell death research: Five riddles. Cell 177, 1094–1107 (2019). https://doi.org/10.1016/j.cell.2019.04.024
T.L. Aaes, A. Kaczmarek, T. Delvaeye, B. De Craene, S. De Koker, L. Heyndrickx, I. Delrue, J. Taminau, B. Wiernicki, P. De Groote, A.D. Garg, L. Leybaert, J. Grooten, M.J. Bertrand, P. Agostinis, G. Berx, W. Declercq, P. Vandenabeele, D.V. Krysko, Vaccination with Necroptotic Cancer cells induces efficient anti-tumor immunity. Cell Rep 15, 274–287 (2016). https://doi.org/10.1016/j.celrep.2016.03.037
N. Casares, M.O. Pequignot, A. Tesniere, F. Ghiringhelli, S. Roux, N. Chaput, E. Schmitt, A. Hamai, S. Hervas-Stubbs, M. Obeid, F. Coutant, D. Metivier, E. Pichard, P. Aucouturier, G. Pierron, C. Garrido, L. Zitvogel, G. Kroemer, Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 202, 1691–1701 (2005). https://doi.org/10.1084/jem.20050915
H. Yang, Y. Ma, G. Chen, H. Zhou, T. Yamazaki, C. Klein, F. Pietrocola, E. Vacchelli, S. Souquere, A. Sauvat, L. Zitvogel, O. Kepp, G. Kroemer, Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology 5, e1149673 (2016). https://doi.org/10.1080/2162402X.2016.1149673
L. Galluzzi, A. Buque, O. Kepp, L. Zitvogel, G. Kroemer, Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17, 97–111 (2017). https://doi.org/10.1038/nri.2016.107
O. Kepp, L. Senovilla, I. Vitale, E. Vacchelli, S. Adjemian, P. Agostinis, L. Apetoh, F. Aranda, V. Barnaba, N. Bloy, L. Bracci, K. Breckpot, D. Brough, A. Buque, M.G. Castro, M. Cirone, M.I. Colombo, I. Cremer, S. Demaria, L. Dini, A.G. Eliopoulos, A. Faggioni, S.C. Formenti, J. Fucikova, L. Gabriele, U.S. Gaipl, J. Galon, A. Garg, F. Ghiringhelli, N.A. Giese, Z.S. Guo, A. Hemminki, M. Herrmann, J.W. Hodge, S. Holdenrieder, J. Honeychurch, H.M. Hu, X. Huang, T.M. Illidge, K. Kono, M. Korbelik, D.V. Krysko, S. Loi, P.R. Lowenstein, E. Lugli, Y. Ma, F. Madeo, A.A. Manfredi, I. Martins, D. Mavilio, L. Menger, N. Merendino, M. Michaud, G. Mignot, K.L. Mossman, G. Multhoff, R. Oehler, F. Palombo, T. Panaretakis, J. Pol, E. Proietti, J.E. Ricci, C. Riganti, P. Rovere-Querini, A. Rubartelli, A. Sistigu, M.J. Smyth, J. Sonnemann, R. Spisek, J. Stagg, A.Q. Sukkurwala, E. Tartour, A. Thorburn, S.H. Thorne, P. Vandenabeele, F. Velotti, S.T. Workenhe, H. Yang, W.X. Zong, L. Zitvogel, G. Kroemer, L. Galluzzi, Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 3, e955691 (2014). https://doi.org/10.4161/21624011.2014.955691
M. Diederich, Natural compound inducers of immunogenic cell death. Arch Pharm Res 42, 629–645 (2019). https://doi.org/10.1007/s12272-019-01150-z
J. Zhou, G. Wang, Y. Chen, H. Wang, Y. Hua, Z. Cai, Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med 23, 4854–4865 (2019). https://doi.org/10.1111/jcmm.14356
A.D. Garg, P. Agostinis, Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev 280, 126–148 (2017). https://doi.org/10.1111/imr.12574
M. Obeid, A. Tesniere, F. Ghiringhelli, G.M. Fimia, L. Apetoh, J.L. Perfettini, M. Castedo, G. Mignot, T. Panaretakis, N. Casares, D. Metivier, N. Larochette, P. van Endert, F. Ciccosanti, M. Piacentini, L. Zitvogel, G. Kroemer, Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13, 54–61 (2007). https://doi.org/10.1038/nm1523
I. Martins, Y. Wang, M. Michaud, Y. Ma, A.Q. Sukkurwala, S. Shen, O. Kepp, D. Metivier, L. Galluzzi, J.L. Perfettini, L. Zitvogel, G. Kroemer, Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 21, 79–91 (2014). https://doi.org/10.1038/cdd.2013.75
A.D. Garg, D.V. Krysko, P. Vandenabeele, P. Agostinis, Extracellular ATP and P(2)X(7) receptor exert context-specific immunogenic effects after immunogenic cancer cell death. Cell Death Dis 7, e2097 (2016). https://doi.org/10.1038/cddis.2015.411
P. Scaffidi, T. Misteli, M.E. Bianchi, Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002). https://doi.org/10.1038/nature00858
A.D. Garg, L. Galluzzi, L. Apetoh, T. Baert, R.B. Birge, J.M.B.-S. Pedro, K. Breckpot, D. Brough, R. Chaurio, M. Cirone, A. Coosemans, P.G. Coulie, D. De Ruysscher, L. Dini, P. de Witte, A.M. Dudek-Peric, A. Faggioni, J. Fucikova, U.S. Gaipl, J. Golab, M.L. Gougeon, M.R. Hamblin, A. Hemminki, M. Herrmann, J.W. Hodge, O. Kepp, G. Kroemer, D.V. Krysko, W.G. Land, F. Madeo, A.A. Manfredi, S.R. Mattarollo, C. Maueroder, N. Merendino, G. Multhoff, T. Pabst, J.E. Ricci, C. Riganti, E. Romano, N. Rufo, M.J. Smyth, J. Sonnemann, R. Spisek, J. Stagg, E. Vacchelli, P. Vandenabeele, L. Vandenberk, B.J. Van den Eynde, S. Van Gool, F. Velotti, L. Zitvogel, P. Agostinis, Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front Immunol 6, 588 (2015). https://doi.org/10.3389/fimmu.2015.00588
A.D. Garg, A.M. Dudek-Peric, E. Romano, P. Agostinis, Immunogenic cell death. Int J Dev Biol 59, 131–140 (2015). https://doi.org/10.1387/ijdb.150061pa
A. Almanza, A. Carlesso, C. Chintha, S. Creedican, D. Doultsinos, B. Leuzzi, A. Luis, N. McCarthy, L. Montibeller, S. More, A. Papaioannou, F. Puschel, M.L. Sassano, J. Skoko, P. Agostinis, J. de Belleroche, L.A. Eriksson, S. Fulda, A.M. Gorman, S. Healy, A. Kozlov, C. Munoz-Pinedo, M. Rehm, E. Chevet, A. Samali, Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J 286, 241–278 (2019). https://doi.org/10.1111/febs.14608
C. Hetz, F.R. Papa, The unfolded protein response and cell fate control. Mol Cell 69, 169–181 (2018). https://doi.org/10.1016/j.molcel.2017.06.017
L. Bezu, A. Sauvat, J. Humeau, L.C. Gomes-da-Silva, K. Iribarren, S. Forveille, P. Garcia, L. Zhao, P. Liu, L. Zitvogel, L. Senovilla, O. Kepp, G. Kroemer, eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ 25, 1375–1393 (2018). https://doi.org/10.1038/s41418-017-0044-9
T. Panaretakis, O. Kepp, U. Brockmeier, A. Tesniere, A.C. Bjorklund, D.C. Chapman, M. Durchschlag, N. Joza, G. Pierron, P. van Endert, J. Yuan, L. Zitvogel, F. Madeo, D.B. Williams, G. Kroemer, Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 28, 578–590 (2009). https://doi.org/10.1038/emboj.2009.1
P. Giglio, M. Gagliardi, N. Tumino, F. Antunes, S. Smaili, D. Cotella, C. Santoro, R. Bernardini, M. Mattei, M. Piacentini, M. Corazzari, PKR and GCN2 stress kinases promote an ER stress-independent eIF2alpha phosphorylation responsible for calreticulin exposure in melanoma cells. Oncoimmunology 7, e1466765 (2018). https://doi.org/10.1080/2162402X.2018.1466765
Global Burden of Disease Cancer Collaboration, C. Fitzmaurice, D. Abate, N. Abbasi, H. Abbastabar, F. Abd-Allah, O. Abdel-Rahman, A. Abdelalim, A. Abdoli, I. Abdollahpour, A.S.M. Abdulle, N.D. Abebe, H.N. Abraha, L.J. Abu-Raddad, A. Abualhasan, I.A. Adedeji, S.M. Advani, M. Afarideh, M. Afshari, M. Aghaali, D. Agius, S. Agrawal, A. Ahmadi, E. Ahmadian, E. Ahmadpour, M.B. Ahmed, M.E. Akbari, T. Akinyemiju, Z. Al-Aly, A.M. AlAbdulKader, F. Alahdab, T. Alam, G.M. Alamene, B.T.T. Alemnew, K.A. Alene, C. Alinia, V. Alipour, S.M. Aljunid, F.A. Bakeshei, M.A.H. Almadi, A. Almasi-Hashiani, U. Alsharif, S. Alsowaidi, N. Alvis-Guzman, E. Amini, S. Amini, Y.A. Amoako, Z. Anbari, N.H. Anber, C.L. Andrei, M. Anjomshoa, F. Ansari, A. Ansariadi, S.C.Y. Appiah, M. Arab-Zozani, J. Arabloo, Z. Arefi, O. Aremu, H.A. Areri, A. Artaman, H. Asayesh, E.T. Asfaw, A.F. Ashagre, R. Assadi, B. Ataeinia, H.T. Atalay, Z. Ataro, S. Atique, M. Ausloos, L. Avila-Burgos, E. Avokpaho, A. Awasthi, N. Awoke, B.P. Ayala Quintanilla, M.A. Ayanore, H.T. Ayele, E. Babaee, U. Bacha, A. Badawi, M. Bagherzadeh, E. Bagli, S. Balakrishnan, A. Balouchi, T.W. Barnighausen, R.J. Battista, M. Behzadifar, M. Behzadifar, B.B. Bekele, Y.B. Belay, Y.M. Belayneh, K.K.S. Berfield, A. Berhane, E. Bernabe, M. Beuran, N. Bhakta, K. Bhattacharyya, B. Biadgo, A. Bijani, M.S. Bin Sayeed, C. Birungi, C. Bisignano, H. Bitew, T. Bjorge, A. Bleyer, K.A. Bogale, H.A. Bojia, A.M. Borzi, C. Bosetti, I.R. Bou-Orm, H. Brenner, J.D. Brewer, A.N. Briko, N.I. Briko, M.T. Bustamante-Teixeira, Z.A. Butt, G. Carreras, J.J. Carrero, F. Carvalho, C. Castro, F. Castro, F. Catala-Lopez, E. Cerin, Y. Chaiah, W.F. Chanie, V.K. Chattu, P. Chaturvedi, N.S. Chauhan, M. Chehrazi, P.P. Chiang, T.Y. Chichiabellu, O.G. Chido-Amajuoyi, O. Chimed-Ochir, J.J. Choi, D.J. Christopher, D.T. Chu, M.M. Constantin, V.M. Costa, E. Crocetti, C.S. Crowe, M.P. Curado, S.M.A. Dahlawi, G. Damiani, A.H. Darwish, A. Daryani, J. das Neves, F.M. Demeke, A.B. Demis, B.W. Demissie, G.T. Demoz, E. Denova-Gutierrez, A. Derakhshani, K.S. Deribe, R. Desai, B.B. Desalegn, M. Desta, S. Dey, S.D. Dharmaratne, M. Dhimal, D. Diaz, M.T.T. Dinberu, S. Djalalinia, D.T. Doku, T.M. Drake, M. Dubey, E. Dubljanin, E.E. Duken, H. Ebrahimi, A. Effiong, A. Eftekhari, I. El Sayed, M.E.S. Zaki, S.I. El-Jaafary, Z. El-Khatib, D.A. Elemineh, H. Elkout, R.G. Ellenbogen, A. Elsharkawy, M.H. Emamian, D.A. Endalew, A.Y. Endries, B. Eshrati, I. Fadhil, V. Fallah, M. Faramarzi, M.A. Farhangi, A. Farioli, F. Farzadfar, N. Fentahun, E. Fernandes, G.T. Feyissa, I. Filip, F. Fischer, J.L. Fisher, L.M. Force, M. Foroutan, M. Freitas, T. Fukumoto, N.D. Futran, S. Gallus, F.G. Gankpe, R.T. Gayesa, T.T. Gebrehiwot, G.G. Gebremeskel, G.A. Gedefaw, B.K. Gelaw, B. Geta, S. Getachew, K.E. Gezae, M. Ghafourifard, A. Ghajar, A. Ghashghaee, A. Gholamian, P.S. Gill, T.T.G. Ginindza, A. Girmay, M. Gizaw, R.S. Gomez, S.V. Gopalani, G. Gorini, B.N.G. Goulart, A. Grada, M. Ribeiro Guerra, A.L.S. Guimaraes, P.C. Gupta, R. Gupta, K. Hadkhale, A. Haj-Mirzaian, A. Haj-Mirzaian, R.R. Hamadeh, S. Hamidi, L.K. Hanfore, J.M. Haro, M. Hasankhani, A. Hasanzadeh, H.Y. Hassen, R.J. Hay, S.I. Hay, A. Henok, N.J. Henry, C. Herteliu, H.D. Hidru, C.L. Hoang, M.K. Hole, P. Hoogar, N. Horita, H.D. Hosgood, M. Hosseini, M. Hosseinzadeh, M. Hostiuc, S. Hostiuc, M. Househ, M.M. Hussen, B. Ileanu, M.D. Ilic, K. Innos, S.S.N. Irvani, K.R. Iseh, S.M.S. Islam, F. Islami, N. Jafari Balalami, M. Jafarinia, L. Jahangiry, M.A. Jahani, N. Jahanmehr, M. Jakovljevic, S.L. James, M. Javanbakht, S. Jayaraman, S.H. Jee, E. Jenabi, R.P. Jha, J.B. Jonas, J. Jonnagaddala, T. Joo, S.B. Jungari, M. Jurisson, A. Kabir, F. Kamangar, A. Karch, N. Karimi, A. Karimian, A. Kasaeian, G.G. Kasahun, B. Kassa, T.D. Kassa, M.W. Kassaw, A. Kaul, P.N. Keiyoro, A.G. Kelbore, A.A. Kerbo, Y.S. Khader, M. Khalilarjmandi, E.A. Khan, G. Khan, Y.H. Khang, K. Khatab, A. Khater, M. Khayamzadeh, M. Khazaee-Pool, S. Khazaei, A.T. Khoja, M.H. Khosravi, J. Khubchandani, N. Kianipour, D. Kim, Y.J. Kim, A. Kisa, S. Kisa, K. Kissimova-Skarbek, H. Komaki, A. Koyanagi, K.J. Krohn, B.K. Bicer, N. Kugbey, V. Kumar, D. Kuupiel, C. La Vecchia, D.P. Lad, E.A. Lake, A.M. Lakew, D.K. Lal, F.H. Lami, Q. Lan, S. Lasrado, P. Lauriola, J.V. Lazarus, J. Leigh, C.T. Leshargie, Y. Liao, M.A. Limenih, S. Listl, A.D. Lopez, P.D. Lopukhov, R. Lunevicius, M. Madadin, S. Magdeldin, H.M.A. El Razek, A. Majeed, A. Maleki, R. Malekzadeh, A. Manafi, N. Manafi, W.A. Manamo, M. Mansourian, M.A. Mansournia, L.G. Mantovani, S. Maroufizadeh, S.M.S. Martini, T.P. Mashamba-Thompson, B.B. Massenburg, M.T. Maswabi, M.R. Mathur, C. McAlinden, M. McKee, H.A.A. Meheretu, R. Mehrotra, V. Mehta, T. Meier, Y.A. Melaku, G.G. Meles, H.G. Meles, A. Melese, M. Melku, P.T.N. Memiah, W. Mendoza, R.G. Menezes, S. Merat, T.J. Meretoja, T. Mestrovic, B. Miazgowski, T. Miazgowski, K.M.M. Mihretie, T.R. Miller, E.J. Mills, S.M. Mir, H. Mirzaei, H.R. Mirzaei, R. Mishra, B. Moazen, D.K. Mohammad, K.A. Mohammad, Y. Mohammad, A.M. Darwesh, A. Mohammadbeigi, H. Mohammadi, M. Mohammadi, M. Mohammadian, A. Mohammadian-Hafshejani, M. Mohammadoo-Khorasani, R. Mohammadpourhodki, A.S. Mohammed, J.A. Mohammed, S. Mohammed, F. Mohebi, A.H. Mokdad, L. Monasta, Y. Moodley, M. Moosazadeh, M. Moossavi, G. Moradi, M. Moradi-Joo, M. Moradi-Lakeh, F. Moradpour, L. Morawska, J. Morgado-da-Costa, N. Morisaki, S.D. Morrison, A. Mosapour, S.M. Mousavi, A.A. Muche, O.S.S. Muhammed, J. Musa, A.R. Nabhan, M. Naderi, A.J. Nagarajan, G. Nagel, A. Nahvijou, G. Naik, F. Najafi, L. Naldi, H.S. Nam, N. Nasiri, J. Nazari, I. Negoi, S. Neupane, P.A. Newcomb, H.A. Nggada, J.W. Ngunjiri, C.T. Nguyen, L. Nikniaz, D.N.A. Ningrum, Y.L. Nirayo, M.R. Nixon, C.A. Nnaji, M. Nojomi, S. Nosratnejad, M.N. Shiadeh, M.S. Obsa, R. Ofori-Asenso, F.A. Ogbo, I.H. Oh, A.T. Olagunju, T.O. Olagunju, M.M. Oluwasanu, A.E. Omonisi, O.E. Onwujekwe, A.M. Oommen, E. Oren, D.D.V. Ortega-Altamirano, E. Ota, S.S. Otstavnov, M.O. Owolabi, A. M. P, J.R. Padubidri, S. Pakhale, A.H. Pakpour, A. Pana, E.K. Park, H. Parsian, T. Pashaei, S. Patel, S.T. Patil, A. Pennini, D.M. Pereira, C. Piccinelli, J.D. Pillay, M. Pirestani, F. Pishgar, M.J. Postma, H. Pourjafar, F. Pourmalek, A. Pourshams, S. Prakash, N. Prasad, M. Qorbani, M. Rabiee, N. Rabiee, A. Radfar, A. Rafiei, F. Rahim, M. Rahimi, M.A. Rahman, F. Rajati, S.M. Rana, S. Raoofi, G.K. Rath, D.L. Rawaf, S. Rawaf, R.C. Reiner, A.M.N. Renzaho, N. Rezaei, A. Rezapour, A.I. Ribeiro, D. Ribeiro, L. Ronfani, E.M. Roro, G. Roshandel, A. Rostami, R.S. Saad, P. Sabbagh, S. Sabour, B. Saddik, S. Safiri, A. Sahebkar, M.R. Salahshoor, F. Salehi, H. Salem, M.R. Salem, H. Salimzadeh, J.A. Salomon, A.M. Samy, J. Sanabria, M.M. Santric Milicevic, B. Sartorius, A. Sarveazad, B. Sathian, M. Satpathy, M. Savic, M. Sawhney, M. Sayyah, I.J.C. Schneider, B. Schottker, M. Sekerija, S.G. Sepanlou, M. Sepehrimanesh, S. Seyedmousavi, F. Shaahmadi, H. Shabaninejad, M. Shahbaz, M.A. Shaikh, A. Shamshirian, M. Shamsizadeh, H. Sharafi, Z. Sharafi, M. Sharif, A. Sharifi, H. Sharifi, R. Sharma, A. Sheikh, R. Shirkoohi, S.R. Shukla, S. Si, S. Siabani, D.A.S. Silva, D.G.A. Silveira, A. Singh, J.A. Singh, S. Sisay, F. Sitas, E. Sobngwi, M. Soofi, J.B. Soriano, V. Stathopoulou, M.B. Sufiyan, R. Tabares-Seisdedos, T. Tabuchi, K. Takahashi, O.R. Tamtaji, M.R. Tarawneh, S.G. Tassew, P. Taymoori, A. Tehrani-Banihashemi, M.H. Temsah, O. Temsah, B.E. Tesfay, F.H. Tesfay, M.Y. Teshale, G.A. Tessema, S. Thapa, K.G. Tlaye, R. Topor-Madry, M.R. Tovani-Palone, E. Traini, B.X. Tran, K.B. Tran, A.G. Tsadik, I. Ullah, O.A. Uthman, M. Vacante, M. Vaezi, P. Varona Perez, Y. Veisani, S. Vidale, F.S. Violante, V. Vlassov, S.E. Vollset, T. Vos, K. Vosoughi, G.T. Vu, I.S. Vujcic, H. Wabinga, T.M. Wachamo, F.S. Wagnew, Y. Waheed, F. Weldegebreal, G.T. Weldesamuel, T. Wijeratne, D.Z. Wondafrash, T.E. Wonde, A.B. Wondmieneh, H.M. Workie, R. Yadav, A. Yadegar, A. Yadollahpour, M. Yaseri, V. Yazdi-Feyzabadi, A. Yeshaneh, M.A. Yimam, E.M. Yimer, E. Yisma, N. Yonemoto, M.Z. Younis, B. Yousefi, M. Yousefifard, C. Yu, E. Zabeh, V. Zadnik, T.Z. Moghadam, Z. Zaidi, M. Zamani, H. Zandian, A. Zangeneh, L. Zaki, K. Zendehdel, Z.M. Zenebe, T.A. Zewale, A. Ziapour, S. Zodpey and C.J.L. Murray, Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 5, 1749–1768 (2019). doi: https://doi.org/10.1001/jamaoncol.2019.2996
J. Zhou, T. Yang, L. Liu, B. Lu, Chemotherapy oxaliplatin sensitizes prostate cancer to immune checkpoint blockade therapies via stimulating tumor immunogenicity. Mol Med Rep 16, 2868–2874 (2017). https://doi.org/10.3892/mmr.2017.6908
J. Kapuscinski, Z. Darzynkiewicz, Interactions of antitumor agents Ametantrone and Mitoxantrone (Novatrone) with double-stranded DNA. Biochem Pharmacol 34, 4203–4213 (1985). https://doi.org/10.1016/0006-2952(85)90275-8
J.S. de Bono, S. Oudard, M. Ozguroglu, S. Hansen, J.P. Machiels, I. Kocak, G. Gravis, I. Bodrogi, M.J. Mackenzie, L. Shen, M. Roessner, S. Gupta, A.O. Sartor, T. Investigators, Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 376, 1147–1154 (2010). https://doi.org/10.1016/S0140-6736(10)61389-X
G. Gravis, Systemic treatment for metastatic prostate cancer. Asian J Urol 6, 162–168 (2019). https://doi.org/10.1016/j.ajur.2019.02.002
J. Qin, N.M. Kunda, G. Qiao, K. Tulla, B.S. Prabhakar, A.V. Maker, Vaccination with Mitoxantrone-treated primary Colon Cancer cells enhances tumor-infiltrating lymphocytes and clinical responses in colorectal liver metastases. J Surg Res 233, 57–64 (2019). https://doi.org/10.1016/j.jss.2018.07.068
T. Colangelo, G. Polcaro, P. Ziccardi, L. Muccillo, M. Galgani, B. Pucci, M.R. Milone, A. Budillon, M. Santopaolo, G. Mazzoccoli, G. Matarese, L. Sabatino, V. Colantuoni, The miR-27a-calreticulin axis affects drug-induced immunogenic cell death in human colorectal cancer cells. Cell Death Dis 7, e2108 (2016). https://doi.org/10.1038/cddis.2016.29
C. Li, C. He, Y. Xu, H. Xu, Y. Tang, H. Chavan, S. Duan, A. Artigues, M.L. Forrest, P. Krishnamurthy, S. Han, J.M. Holzbeierlein, B. Li, Alternol eliminates excessive ATP production by disturbing Krebs cycle in prostate cancer. Prostate 79, 628–639 (2019). https://doi.org/10.1002/pros.23767
C. Li, W. Jiang, Q. Hu, L.C. Li, L. Dong, R. Chen, Y. Zhang, Y. Tang, J.B. Thrasher, C.B. Liu, B. Li, Enhancing DPYSL3 gene expression via a promoter-targeted small activating RNA approach suppresses cancer cell motility and metastasis, Oncotarget 7, 22893–22910 (2016). https://doi.org/10.18632/oncotarget.8290
K. Berns, E.M. Hijmans, J. Mullenders, T.R. Brummelkamp, A. Velds, M. Heimerikx, R.M. Kerkhoven, M. Madiredjo, W. Nijkamp, B. Weigelt, R. Agami, W. Ge, G. Cavet, P.S. Linsley, R.L. Beijersbergen, R. Bernards, A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428, 431–437 (2004). https://doi.org/10.1038/nature02371
M. Soto, J.A. Raaijmakers, B. Bakker, D.C.J. Spierings, P.M. Lansdorp, F. Foijer, R.H. Medema, p53 prohibits propagation of chromosome segregation errors that produce structural aneuploidies. Cell Rep 19, 2423–2431 (2017). https://doi.org/10.1016/j.celrep.2017.05.055
C. Li, H. Xu, L. Xiao, H. Zhu, G. Zhang, W. Wei, K. Li, X. Cao, D. Shen, J. Holzbeierlein, B. Li, CRMP4a suppresses cell motility by sequestering RhoA activity in prostate cancer cells. Cancer Biol Ther 19, 1193–1203 (2018). https://doi.org/10.1080/15384047.2018.1491507
C. Pozzi, A. Cuomo, I. Spadoni, E. Magni, A. Silvola, A. Conte, S. Sigismund, P.S. Ravenda, T. Bonaldi, M.G. Zampino, C. Cancelliere, P.P. Di Fiore, A. Bardelli, G. Penna, M. Rescigno, The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med 22, 624–631 (2016). https://doi.org/10.1038/nm.4078
G.H. Nam, E.J. Lee, Y.K. Kim, Y. Hong, Y. Choi, M.J. Ryu, J. Woo, Y. Cho, D.J. Ahn, Y. Yang, I.C. Kwon, S.Y. Park, I.S. Kim, Combined rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer. Nat Commun 9, 2165 (2018). https://doi.org/10.1038/s41467-018-04607-9
A. Khan, O. Fornes, A. Stigliani, M. Gheorghe, J.A. Castro-Mondragon, R. van der Lee, A. Bessy, J. Cheneby, S.R. Kulkarni, G. Tan, D. Baranasic, D.J. Arenillas, A. Sandelin, K. Vandepoele, B. Lenhard, B. Ballester, W.W. Wasserman, F. Parcy, A. Mathelier, JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res 46, D1284 (2018). https://doi.org/10.1093/nar/gkx1188
D. Schmidt, M.D. Wilson, C. Spyrou, G.D. Brown, J. Hadfield, D.T. Odom, ChIP-seq: Using high-throughput sequencing to discover protein-DNA interactions. Methods 48, 240–248 (2009). https://doi.org/10.1016/j.ymeth.2009.03.001
C.P. Bergstrom, B. Ruffell, C.M. Ho, C.S. Higano, W.J. Ellis, M. Garzotto, T.M. Beer, J.N. Graff, Docetaxel and mitoxantrone before radical prostatectomy in men with high-risk prostate cancer: 10-year follow-up and immune correlates. Anti-Cancer Drugs 28, 120–126 (2017). https://doi.org/10.1097/CAD.0000000000000438
C. Sanchez, P. Mendoza, H.R. Contreras, J. Vergara, J.A. McCubrey, C. Huidobro, E.A. Castellon, Expression of multidrug resistance proteins in prostate cancer is related with cell sensitivity to chemotherapeutic drugs. Prostate 69, 1448–1459 (2009). https://doi.org/10.1002/pros.20991
L. Bezu, L.C. Gomes-de-Silva, H. Dewitte, K. Breckpot, J. Fucikova, R. Spisek, L. Galluzzi, O. Kepp, G. Kroemer, Combinatorial strategies for the induction of immunogenic cell death. Front Immunol 6, 187 (2015). https://doi.org/10.3389/fimmu.2015.00187
R.C. Wek, Role of eIF2alpha kinases in translational control and adaptation to cellular stress. Cold Spring Harb Perspect Biol 10 (2018). https://doi.org/10.1101/cshperspect.a032870
A. Rashidi, J. Miska, C. Lee-Chang, D. Kanojia, W.K. Panek, A. Lopez-Rosas, P. Zhang, Y. Han, T. Xiao, K.C. Pituch, J.W. Kim, M. Talebian, J. Fares, M.S. Lesniak, GCN2 is essential for CD8(+) T cell survival and function in murine models of malignant glioma. Cancer Immunol Immunother 69, 81–94 (2020). https://doi.org/10.1007/s00262-019-02441-6
P.R. Romano, M.T. Garcia-Barrio, X. Zhang, Q. Wang, D.R. Taylor, F. Zhang, C. Herring, M.B. Mathews, J. Qin, A.G. Hinnebusch, Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol 18, 2282–2297 (1998). https://doi.org/10.1128/mcb.18.4.2282
H.P. Harding, Y. Zhang, A. Bertolotti, H. Zeng, D. Ron, Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5, 897–904 (2000). https://doi.org/10.1016/s1097-2765(00)80330-5
G. Guo, M. Yu, W. Xiao, E. Celis, Y. Cui, Local activation of p53 in the tumor microenvironment overcomes immune suppression and enhances antitumor immunity. Cancer Res 77, 2292–2305 (2017). https://doi.org/10.1158/0008-5472.CAN-16-2832
S. Bottger, E. Jerszyk, B. Low, C. Walker, Genotoxic stress-induced expression of p53 and apoptosis in leukemic clam hemocytes with cytoplasmically sequestered p53. Cancer Res 68, 777–782 (2008). https://doi.org/10.1158/0008-5472.CAN-06-0968
A.G. Carroll, H.J. Voeller, L. Sugars, E.P. Gelmann, p53 oncogene mutations in three human prostate cancer cell lines. Prostate 23, 123–134 (1993). https://doi.org/10.1002/pros.2990230206
M. Michaud, I. Martins, A.Q. Sukkurwala, S. Adjemian, Y. Ma, P. Pellegatti, S. Shen, O. Kepp, M. Scoazec, G. Mignot, S. Rello-Varona, M. Tailler, L. Menger, E. Vacchelli, L. Galluzzi, F. Ghiringhelli, F. di Virgilio, L. Zitvogel, G. Kroemer, Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011). https://doi.org/10.1126/science.1208347
J.S. Park, F. Gamboni-Robertson, Q. He, D. Svetkauskaite, J.Y. Kim, D. Strassheim, J.W. Sohn, S. Yamada, I. Maruyama, A. Banerjee, A. Ishizaka, E. Abraham, High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol Cell Physiol 290, C917–C924 (2006). https://doi.org/10.1152/ajpcell.00401.2005
I. Martins, A. Tesniere, O. Kepp, M. Michaud, F. Schlemmer, L. Senovilla, C. Seror, D. Metivier, J.L. Perfettini, L. Zitvogel, G. Kroemer, Chemotherapy induces ATP release from tumor cells. Cell Cycle 8, 3723–3728 (2009). https://doi.org/10.4161/cc.8.22.10026
A.D. Garg, D.V. Krysko, T. Verfaillie, A. Kaczmarek, G.B. Ferreira, T. Marysael, N. Rubio, M. Firczuk, C. Mathieu, A.J. Roebroek, W. Annaert, J. Golab, P. de Witte, P. Vandenabeele, P. Agostinis, A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 31, 1062–1079 (2012). https://doi.org/10.1038/emboj.2011.497
L. Bezu, A. Sauvat, J. Humeau, M. Leduc, O. Kepp, G. Kroemer, eIF2alpha phosphorylation: A hallmark of immunogenic cell death. Oncoimmunology 7, e1431089 (2018). https://doi.org/10.1080/2162402X.2018.1431089
J. Han, S.H. Back, J. Hur, Y.H. Lin, R. Gildersleeve, J. Shan, C.L. Yuan, D. Krokowski, S. Wang, M. Hatzoglou, M.S. Kilberg, M.A. Sartor, R.J. Kaufman, ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15, 481–490 (2013). https://doi.org/10.1038/ncb2738
S.M. Reed, D.E. Quelle, p53 acetylation: Regulation and consequences. Cancers (Basel) 7, 30–69 (2014). https://doi.org/10.3390/cancers7010030
M.F. Lavin, N. Gueven, The complexity of p53 stabilization and activation. Cell Death Differ 13, 941–950 (2006). https://doi.org/10.1038/sj.cdd.4401925
Y. Xia, R.C. Padre, T.H. De Mendoza, V. Bottero, V.B. Tergaonkar, I.M. Verma, Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci U S A 106, 2629–2634 (2009). https://doi.org/10.1073/pnas.0812256106
G.S. Ivanov, T. Ivanova, J. Kurash, A. Ivanov, S. Chuikov, F. Gizatullin, E.M. Herrera-Medina, F. Rauscher 3rd, D. Reinberg, N.A. Barlev, Methylation-acetylation interplay activates p53 in response to DNA damage. Mol Cell Biol 27, 6756–6769 (2007). https://doi.org/10.1128/MCB.00460-07
J. Huang, L. Perez-Burgos, B.J. Placek, R. Sengupta, M. Richter, J.A. Dorsey, S. Kubicek, S. Opravil, T. Jenuwein, S.L. Berger, Repression of p53 activity by Smyd2-mediated methylation. Nature 444, 629–632 (2006). https://doi.org/10.1038/nature05287
S.F. Slovin, Immunotherapy for castration-resistant prostate cancer: Has its time arrived? Expert Opin Biol Ther 20, 481–487 (2020). https://doi.org/10.1080/14712598.2020.1735345
I. Vanmeerbeek, J. Sprooten, D. De Ruysscher, S. Tejpar, P. Vandenberghe, J. Fucikova, R. Spisek, L. Zitvogel, G. Kroemer, L. Galluzzi, A.D. Garg, Trial watch: Chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 9, 1703449 (2020). https://doi.org/10.1080/2162402X.2019.1703449
J.W. Hodge, C.T. Garnett, B. Farsaci, C. Palena, K.Y. Tsang, S. Ferrone, S.R. Gameiro, Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer 133, 624–636 (2013). https://doi.org/10.1002/ijc.28070
Acknowledgments
This work was partially supported by the Shandong Provincial Natural Science Foundation of China (ZR2016HL25 and ZR2016CQ01), NSFC grant 81501770 and an internal grant from Jining Medical University (JYHL2018ZD02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
All protocols and procedures in this study were approved by the institutional review committee of Jining Medical University for animal welfare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 34 kb)
ESM 2
MTX induces apoptosis in prostate cancer cells. a. LNCaP and 22RV1 cells were treated with the vehicle or MTX (1 μM) for 20 h and then harvested for apoptosis analysis using Annexin-V/PI staining approach coupled with flow cytometry. b. Quantitative data of apoptosis were presented from three independent experiments. Early apoptotic was defined as Annexin positive population, later apoptotic as Annexin/PI positive and necrotic death as PI positive only. The asterisks indicate a significant difference compared to the vehicle control (Student t-test, *p < 0.05, **p < 0.01, ***p < 0.001). c. LNCaP and 22RV1 cells were treated with the vehicle or MTX (1 μM) for 20 h and then whole cell proteins were extracted for western blot assay with PARP antibodies. Actin blot served as protein loading control. Data were representative of two independent experiments. (PNG 70 kb)
ESM 3
Effect of PERK or GCN2 Knockdown on MTX-induced apoptosis. LNCaP cells transfected with the shRNAs as indicated were treated with MTX (1 μM) or the vehicle for 20 h, followed by Annexin-V/PI staining, as described earlier. Representative plots of flow cytometry from three independent experiments were shown in panel a. Quantitative data of apoptotic population (%) were summarized in panel b. The asterisks indicate a significant difference compared to the shScr control (Student t-test, *p < 0.05, ***p < 0.001). (PNG 64 kb)
Rights and permissions
About this article
Cite this article
Li, C., Sun, H., Wei, W. et al. Mitoxantrone triggers immunogenic prostate cancer cell death via p53-dependent PERK expression. Cell Oncol. 43, 1099–1116 (2020). https://doi.org/10.1007/s13402-020-00544-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00544-2