Abstract
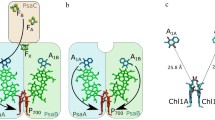
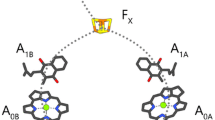
ESEEM measurements were performed at two temperatures on Photosystem I (PS I) complexes depleted of the extrinsic PsaC subunit harboring iron–sulfur 4Fe–4S clusters FA/FB (abbreviated FX-core complexes) embedded in trehalose glassy matrix. The ESEEM modulation frequencies obtained for the FX-core complexes in trehalose matrix were similar at 150 K and 290 K. They correspond to a distance ~ 25 Å between the oxidized primary donor P700·+ and reduced phylloquinone acceptor A1·−. This distance corresponds to the preferential charge separation pathway between P700·+ and A1B·− in the B-branch of the PS I redox cofactors, while the distance estimated previously for the intact PS I complexes (containing the extrinsic PsaC subunit) embedded in dry trehalose matrix was ~ 26 Å, which is typical for the formation of P700·+A1A·− charge-separated radical pairs in the symmetrical A-branch (Sukhanov et al. in Appl Magn Reson 49:1011, 2018). The redirection of electron transfer preferentially from the A to the B-branch of redox cofactors observed in FX-core complexes embedded in dry trehalose matrix can be rationalized by the alteration of the protein domain rigidity/flexibility around phylloquinone molecules in the A1A and A1B sites, which affects the thermodynamics of electron transfer between P700 and A1 in the symmetrical branches of cofactors. This finding of similar modulation frequencies measured for FX-core complexes in trehalose matrix at 150 K and 290 K is opposite to our previously obtained results for intact PS I, where the value of ESEEM modulation frequency markedly decreased upon heating the sample from cryogenic to room temperature (Sukhanov et al. in Appl Magn Reson 49: 1011, 2018). Our comparison of FX-core complexes with intact PS I complexes suggests that 4Fe-4S clusters accelerate spin–lattice relaxation of the unpaired electron spins on the phylloquinone in the A1-site. The result serves as additional argument in favor of the molecular model of the cryoprotective effect of trehalose matrix on the functioning of PS I by way of rigidity changes of the hydrogen-bonding network of the protein with the matrix that is based on the Le Chatelier–Braun principle. According to this thermodynamic principle, changes in both rigidity and flexibility are compensated to establish new thermodynamic equilibria with restored global balance of rigidity and flexibility that is typical within functioning protein complexes.





Similar content being viewed by others
References
A.A. Sukhanov, M.D. Mamedov, K. Möbius, A.Y. Semenov, K.M. Salikhov, Appl. Magn. Reson. 49, 1011 (2018)
P. Jordan, P. Fromme, H.T. Witt, O. Klukas, W. Saenger, N. Krauß, Nature 411, 909 (2001)
G. Palazzo, A. Mallardi, A. Hochkoeppler, L. Cordone, G. Venturoli, Biophys. J. 82, 558 (2002)
F. Francia, M. Malferrari, S. Sacquin-Mora, G. Venturoli, J. Phys. Chem. B 113, 10389 (2009)
M. Malferrari, A. Savitsky, M.D. Mamedov, G.E. Milanovsky, W. Lubitz, K. Möbius, A.Y. Semenov, G. Venturoli, Biochim. Biophys. Acta Bioenerg. 1857, 1440 (2016)
M. Malferrari, A. Nalepa, G. Venturoli, F. Francia, W. Lubitz, K. Möbius, A. Savitsky, Phys. Chem. Chem. Phys. 16, 9831 (2014)
A. Savitsky, O. Gopta, M. Mamedov, J.H. Golbeck, A. Tikhonov, K. Möbius, A. Semenov, Appl. Magn. Reson. 37, 85 (2010)
G. Cottone, S. Giuffrida, S. Bettati, S. Bruno, B. Campanini, M. Marchetti, S. Abbruzzetti, C. Viappiani, A. Cupane, A. Mozzarelli, L. Ronda, Catalysts 9, 1024 (2019)
V. Kurashov, M. Gorka, G.E. Milanovsky, T.W. Johnson, D.A. Cherepanov, A.Y. Semenov, J.H. Golbeck, Biochim. Biophys. Acta Bioenerg. 1859, 1288 (2018)
G. Milanovsky, O. Gopta, A. Petrova, M. Mamedov, M. Gorka, D. Cherepanov, J.H. Golbeck, A. Semenov, Biochim. Biophys. Acta Bioenerg. 1860, 601 (2019)
G.M. Zhidomirov, K.M. Salikhov, Sov. Phys. JETP 29, 1037 (1969)
K.M. Salikhov, S.A. Dzuba, A.M. Raitsimring, J. Magn. Reson. 42, 255 (1981)
T. Li, M.B. Tracka, S. Uddin, J. Casas-Finet, D.J. Jacobs, D.R. Livesay, PLoS ONE 2014, 9 (2014)
G. Shen, J. Zhao, S.K. Reimer, M.L. Antonkine, Q. Cai, S.M. Weiland, J.H. Golbeck, D.A. Bryant, J. Biol. Chem. 277, 20343 (2002)
K.M. Salikhov, I.T. Khairuzhdinov, R.B. Zaripov, Appl. Magn. Reson. 45, 573 (2014)
S. Mula, A. Savitsky, K. Möbius, W. Lubitz, J.H. Golbeck, M.D. Mamedov, A.Y. Semenov, A. Van Der Est, Photochem. Photobiol. Sci. 11, 946 (2012)
M. Plato, N. Krauß, P. Fromme, W. Lubitz, Chem. Phys. 294, 483 (2003)
W. Lubitz, in Photosystem I, ed. by J.H. Golbeck (Springer, Netherlands, 2006), pp. 245–269
V.V. Ptushenko, D.A. Cherepanov, L.I. Krishtalik, A.Y. Semenov, Photosynth. Res. 97, 55 (2008)
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (RFBR). K. M. Salikhov acknowledges RFBR Grant 18-43-160017, A. Yu. Semenov RFBR Grant 17-00-00201. K. Möbius acknowledges sustaining support by the Free University of Berlin, Germany. We want to thank Giovanni Venturoli (University of Bologna, Italy) for stimulating and fruitful discussions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sukhanov, A.A., Mamedov, M.D., Möbius, K. et al. Impact of Iron–Sulfur Clusters on the Spin–Lattice Relaxation Rate and ESEEM Frequency of the Oxidized Primary Donor P700+· and Reduced Phylloquinone Acceptor A1−· in Radical Pairs in Photosystem I Embedded in Trehalose Glassy Matrix. Appl Magn Reson 51, 909–924 (2020). https://doi.org/10.1007/s00723-020-01210-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-020-01210-4