Abstract
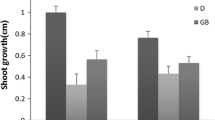
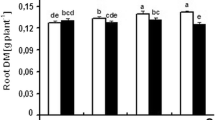
Coix lacryma-jobi L. is an Asian plant widely used in traditional medicine. Drought conditions can lead to an impairment of photosynthesis which can be softened by the exogenous application of proline and γ-aminobutyric acid (GABA) to maintain the electron transport chain (ETC) and avoid reactive oxygen species-induced oxidative damage by inducing antioxidative enzymes. The aim of this study was to evaluate the effects of water deficit on C. lacryma-jobi leaves under the application of proline and GABA. Chlorophyll (Chl) a fluorescence emission, antioxidative enzyme activity (catalase, superoxide dismutase, ascorbate peroxidase and guaiacol peroxidase) and proline and GABA contents in leaf tissues were performed in plants subjected to 12 days of drought. Chl a fluorescence indicated impairments on photosynthetic apparatus due to water deficit that resulted into increased activity of antioxidant enzymes. The application of proline but not GABA was more efficient in alleviating the effects of water deficit, as evidenced by the maintenance of the dynamic dissipation of the photosynthetic energy, besides the greater accumulation of proline under these conditions. Water deficit induces alterations in the ETC, which is softened by the application of proline to cope with the deleterious effects of drought in C. lacryma-jobi plant leaves.



Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- ETC:
-
Electron transport chain
- GABA:
-
γ-Aminobutyric acid
- GPOD:
-
Guaiacol peroxidase
- H2O2 :
-
Hydrogen peroxide
- OEC:
-
Oxygen-evolving complex
- PQ:
-
Plastoquinone
- PQH2 :
-
Plastoquinol
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- QA :
-
Quinone A
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Al-Quraan NA, Al-Share AT (2015) Characterization of the γ-aminobutyric acid shunt pathway and oxidative damage in Arabidopsis thaliana pop 2 mutants under various abiotic stresses. Biol Plant 60:132–138
Azevedo Neto AD, Prisco JT, Eneas Filho J, De Abreu CEB, Gomes Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Bates LS, Waldren RD, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Ben Rejeb K, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284
Blokhina O, Fagerstedt KV (2010) Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems. Physiol Plant 138:447–462
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Corke H, Huang Y, Li JS (2016) Coix: overview. In: Wrigley C, Corke H, Seetharaman K, Faubion J (eds) Encyclopedia of food grains. Academic Press, Oxford, pp 184–189
Dodd IC, Ryan AC (2016) Whole-plant physiological responses to water-deficit stress. eLS. Wiley, Chichester, pp 1–9
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H (2002) Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct Plant Biol 29:461–471
Foyer CH, Ruban AV, Noctor G (2017) Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem J 474:877–883
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Hsiao S, Chen S, Yang I, Chen C, Tsai C, Chuang Y, Wang F, Chen Y, Linc T, Lo Y (2010) Evaluation of plant seedling water stress using 41 dynamic fluorescence index with blue LED-based fluorescence imaging. Comput Electron Agric 72:127–133
Ivanov BN, Borisova-Mubarakhina MM, Kozuleva MA (2018) Formation mechanisms of superoxide radical and hydrogen peroxide in chloroplasts, and factors determining the signalling by hydrogen peroxide. Funct Plant Biol 45:102–110
Johnson BS, Singh NK, Cherry JH, Locy RD (1997) Purification and characterization of glutamate decarboxylase from cowpea. Phytochemistry 46:39–44
Karunamoorthi K, Jegajeevanram K, Vijayalakshmi J, Mengistie E (2013) Traditional medicinal plants: a source of phytotherapeutic modality in resource-constrained healthcare settings. J Evid Bas Complement Altern Med 18:67–74
Kim YH, Kwak SS (2010) The role of antioxidant enzymes during leaf development. In: Gupta SD (ed) Reactive oxygen species and antioxidants in higher plants. Science Publishers, Enfeld, pp 129–150
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Li Y, Tian X, Li S, Chang L, Sun P, Lu Y, Yu X, Chen S, Wu Z, Xua Z, Kang W (2019) Total polysaccharides of adlay bran (Coix lacryma-jobi L.) improve TNF-α induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct 10:2906–2913
Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanisms of stress survival. Antioxid Redox Signal 19:998–1011
Liu X, Huang B (2008) Photosynthetic acclimation to high temperatures associated with heat tolerance in creeping bengrass. J Plant Physiol 165:1947–1953
Osakabe Y, Osakabe K, Shinozaki K, Tran LSP (2014) Response of plants to water stress. Front Plant Sci 5:86
Phukan UJ, Jeena GS, Shukla RK (2016) WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci 7:760
Renault H, Roussel V, Amrani A, Arzel M, Renault D, Boucherdeau A, Deleu C (2010) The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10:20
Ruminta NT, Wicaksono FY (2017) Growth and yield of job’s tears (Coix lacryma-jobi L.) response to different types of oldeman climate classification and row spacing in West Java Indonesia. J Agron 16:76–82
Shanti SS, Karl-Josef D (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Sousa CAF, Sodek L (2003) Alanine metabolism and alanine aminotransferase activity in soybean (Glycine max) during hypoxia of the root system and subsequent return to normoxia. Environ Exp Bot 50:1–8
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications. J Photochem Photobiol B Biol 104:236–257
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 321–362
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of plants vitality: applications in detecting and evaluating the impact of Mycorrhization on host plants. In: Varma A (ed) Mycorrhiza, 3rd edn. Springer, Berlin, pp 679–703
Wang L, Sun J, Yi Q, Wang X, Ju X (2012) Protective effect of polyphenols extract of adlay (Coix lacryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules 17:8886–8897
Yaish MW (2015) Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet Mol Res 14:9943–9950
Yan K, Chen P, Shao H, Shao C, Zhao S, Brestic M (2013) Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 8:62100
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee, Sarin NB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochem Biophys Acta (BBA) Bioenergy 1797:1428–1438
Zargar SM, Gupta N, Nazir MR, Malik FA, Sofi NR, Shikari AB, Salgrota RK (2017) Impact of drought on photosynthesis: molecular perspective. Plant Gene 11:154–159
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Contributions
RAF, JB, CMH, TBM and CRP contributed to the study conception and design. RAF, JB, CMH, ACBC, NPCC, DMC, RPB and CFP were involved in material preparation, data collection and analysis. The first draft of the manuscript was written by RAF, JB and CMH. All authors commented on previous versions of the manuscript, read and approved the final manuscript version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, R.A., Borella, J., Hüther, C.M. et al. Drought-induced stress in leaves of Coix lacryma-jobi L. under exogenous application of proline and GABA amino acids. Braz. J. Bot 43, 513–521 (2020). https://doi.org/10.1007/s40415-020-00637-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-020-00637-0