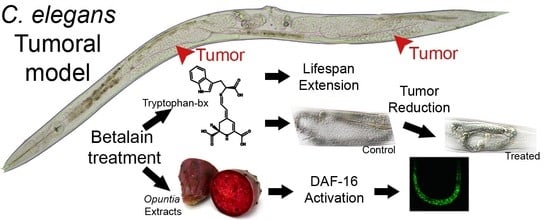
Antitumoral Drug Potential of Tryptophan-Betaxanthin and Related Plant Betalains in the Caenorhabditis elegans Tumoral Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction, Production and Purification of Betalains
2.3. Prickly Pears and Beetroot Extracts
2.4. C. elegans Strains and Culture Conditions
2.5. Germline Tumor Growth Assays
2.6. Betalains and Natural Extracts Treatment in C. elegans
2.7. Antioxidant Activity In Vitro by the ABTS Method
2.8. Antioxidant Activity In Vivo
2.9. Intracellular Localization of daf-16 Transcription Factor
2.10. C. elegans Tumor Induction via Gene Knockdown with RNAi
2.11. Tumor Size Evaluation
2.12. DAPI and Acridine Orange Staining Processes
2.13. Survival Assays
2.14. Statistical Analysis
3. Results and Discussion
3.1. Effects of Natural Extracts on Tumoral C. elegans Strain JK1466
3.2. Natural Extracts Effect on the Lifespan of the Tumoral Worms
3.3. Effect of Pure Betalains in Tumoral C. elegans Strain JK1466
3.4. Survival Assays in the Tumoral Strain JK1466
3.5. Antioxidant Activity
3.6. Pure betalains and Natural Extracts Evaluation as Modulators of the Longevity Pathway
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pratheeshkumar, P.; Son, Y.O.; Korangath, P.; Manu, K.A.; Siveen, K.S. Phytochemicals in cancer prevention and therapy. Biomed. Res. Int. 2015, 2015, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, D.; Arunasree, M.K.; Roy, K.R.; Chandramohan Reddy, T.; Reddy, G.V.; Reddanna, P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia cell line-K562. Phytomedicine 2007, 14, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Abedimanesh, S.; Mesbah Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2018, 8398. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, N.V.; Mani, K.; Fay, D.S. Cancer models in Caenorhabditis elegans. Dev. Dyn. 2010, 239, 1413–1448. [Google Scholar]
- Francis, R.; Barton, M.K.; Kimble, J.; Schedl, T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 1995, 139, 579–606. [Google Scholar]
- Lee, M.-H.; Schedl, T. C. elegans star proteins, GLD-1 and ASD-2, regulate specific RNA targets to control development. In Post-Transcriptional Regulation by STAR Proteins; Springer: Berlin/Heidelberg, Germany, 2010; pp. 106–122. [Google Scholar]
- Ciosk, R.; DePalma, M.; Priess, J.R. Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 2006, 311, 851–853. [Google Scholar] [CrossRef]
- Biedermann, B.; Wright, J.; Senften, M.; Kalchhauser, I.; Sarathy, G.; Lee, M.-H.; Ciosk, R. Translational repression of cyclin E prevents precocious mitosis and embryonic gene activation during C. elegans meiosis. Dev. Cell 2009, 17, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Rubio, M.A.; López-Llorca, R.; Henarejos-Escudero, P.; García-Carmona, F.; Gandía-Herrero, F. Scaled-up biotechnological production of individual betalains in a microbial system. Microb. Biotechnol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Development of a protocol for the semi-synthesis and purification of betaxanthins. Phytochem. Anal. 2006, 17, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Rubio, M.A.; Hernández-García, S.; García-Carmona, F.; Gandía-Herrero, F. Extension of life-span using a RNAi model and in vivo antioxidant effect of Opuntia fruit extracts and pure betalains in Caenorhabditis elegans. Food Chem. 2019, 274, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Rubio, M.A.; Hernández-García, S.; Escribano, J.; Jiménez-Atiénzar, M.; Cabanes, J.; García-Carmona, F.; Gandía-Herrero, F. Betalain health-promoting effects after ingestion in Caenorhabditis elegans are mediated by DAF-16/FOXO and SKN-1/Nrf2 transcription factors. Food Chem. 2020, 330, 127228. [Google Scholar] [CrossRef]
- Ahringer, J. Reverse Genetics. 2006. Available online: http://www.wormbook.org. (accessed on 10 May 2020).
- Gervaise, A.L.; Arur, S. Spatial and temporal analysis of active ERK in the C. elegans germline. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [Green Version]
- Shaham, S. Methods in cell biology. WormBook 2006. [Google Scholar] [CrossRef]
- Lettre, G.; Kritikou, E.A.; Jaeggi, M.; Calixto, A.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Hengartner, M.O. Genome-wide RNAi identifies p53-dependent and -independent regulators of germ cell apoptosis in C. elegans. Cell Death Differ. 2004, 11, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Stroustrup, N.; Ulmschneider, B.E.; Nash, Z.M.; López-Moyado, I.F.; Apfeld, J.; Fontana, W. The Caenorhabditis elegans lifespan machine. Nat. Methods 2013, 10, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.-S.; Lee, S.-J.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef] [Green Version]
- Kapadia, G.J.; Tokuda, H.; Konoshima, T.; Nishino, H. Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract. Cancer Lett. 1996, 100, 211–214. [Google Scholar] [CrossRef]
- Zou, D.; Brewer, M.; Garcia, F.; Feugang, J.M.; Wang, J.; Zang, R.; Liu, H.; Zou, C. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef]
- Lechner, J.F.; Wang, L.-S.; Rocha, C.M.; Larue, B.; Henry, C.; McIntyre, C.M.; Riedl, K.M.; Schwartz, S.J.; Stoner, G.D. Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N -nitrosomethylbenzylamine-treated Rats. J. Med. Food 2010, 13, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Galati, E.M.; Mondello, M.R.; Lauriano, E.R.; Taviano, M.F.; Galluzzo, M.; Miceli, N. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride-induced injury. Phyther. Res. 2005, 19, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.E.; Ciosk, R. RNA-based regulation of pluripotency. Trends Genet. 2013, 29, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Parra, J.; Muñoz, R. Characterization of betacyanin oxidation catalyzed by a peroxidase from Beta vulgaris L. roots. J. Agric. Food Chem. 2001, 49, 4064–4068. [Google Scholar] [CrossRef]
- Guerrero-Rubio, M.A.; Martínez-Zapata, J.; Henarejos-Escudero, P.; García-Carmona, F.; Gandía-Herrero, F. Reversible bleaching of betalains induced by metals and application to the fluorescent determination of anthrax biomarker. Dyes Pigment. 2020, 180, 108493. [Google Scholar] [CrossRef]
- Pourrat, A.; Lejeune, B.; Grand, A.; Pourrat, H. Betalains assay of fermented red beet root extract by high performance liquid chromatography. J. Food Sci. 1988, 53, 294–295. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Jiménez-Atiénzar, M.; Cabanes, J.; García-Carmona, F.; Escribano, J. Stabilization of the bioactive pigment of Opuntia Fruits through maltodextrin encapsulation. J. Agric. Food Chem. 2010, 58, 10646–10652. [Google Scholar] [CrossRef]
- Chen, K.C.; Jian, Y.R.; Sun, M.F.; Chang, T.T.; Lee, C.C.; Chen, C.Y.C. Investigation of silent information regulator 1 (Sirt1) agonists from Traditional Chinese Medicine. J. Biomol. Struct. Dyn. 2013, 31, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Pedreño, M.A.; García-Carmona, F.; Muñoz, R. Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 1998, 9, 124–127. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Cabanes, J.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Encapsulation of the most potent antioxidant betalains in edible matrixes as powders of different colors. J. Agric. Food Chem. 2013, 61, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, phenols and antioxidant capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Purification and antiradical properties of the structural unit of betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Borek, C. Dietary antioxidants and human cancer. Integr. Cancer Ther. 2004, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef]
- Perera, R.M.; Bardeesy, N. When antioxidants are bad. Nature 2011, 475, 43–44. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, R.; Pérez-Villegas, E.M.; Manuel Carrión, Á. AMPK Function in aging process. Curr. Drug Targets 2016, 17, 932–941. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Oh, S.W.; Tissenbaum, H.A. Worming pathways to and from DAF-16/FOXO. Exp. Gerontol. 2006, 41, 928–934. [Google Scholar] [CrossRef]
- Wang, Y.; Oh, S.W.; Deplancke, B.; Luo, J.; Walhout, A.J.M.; Tissenbaum, H.A. C. elegans 14-3-3 proteins regulate life span and interact with SIR-2.1 and DAF-16/FOXO. Mech. Ageing Dev. 2006, 127, 741–747. [Google Scholar] [CrossRef]
- Pinkston, J.M.; Garigan, D.; Hansen, M.; Kenyon, C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 2006, 313, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Pinkston-Gosse, J.; Kenyon, C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat. Genet. 2007, 39, 1403–1409. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henarejos-Escudero, P.; Hernández-García, S.; Guerrero-Rubio, M.A.; García-Carmona, F.; Gandía-Herrero, F. Antitumoral Drug Potential of Tryptophan-Betaxanthin and Related Plant Betalains in the Caenorhabditis elegans Tumoral Model. Antioxidants 2020, 9, 646. https://doi.org/10.3390/antiox9080646
Henarejos-Escudero P, Hernández-García S, Guerrero-Rubio MA, García-Carmona F, Gandía-Herrero F. Antitumoral Drug Potential of Tryptophan-Betaxanthin and Related Plant Betalains in the Caenorhabditis elegans Tumoral Model. Antioxidants. 2020; 9(8):646. https://doi.org/10.3390/antiox9080646
Chicago/Turabian StyleHenarejos-Escudero, Paula, Samanta Hernández-García, M. Alejandra Guerrero-Rubio, Francisco García-Carmona, and Fernando Gandía-Herrero. 2020. "Antitumoral Drug Potential of Tryptophan-Betaxanthin and Related Plant Betalains in the Caenorhabditis elegans Tumoral Model" Antioxidants 9, no. 8: 646. https://doi.org/10.3390/antiox9080646