Abstract
Background
Felbinac trometamol, an anti-inflammatory and analgesic drug, has been used to treat immediate postoperative pain.
Objective
The aim of this study was to evaluate the safety, tolerability, and pharmacokinetics of single or multiple intravenous infusions of felbinac trometamol in healthy Chinese volunteers.
Methods
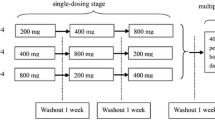
A total of 56 healthy subjects were enrolled in a single-ascending dose study (11.78–377.00 mg), meanwhile 36 subjects were enrolled in a multiple-ascending dose study (47.13–188.50 mg). Safety endpoints included treatment-emergent adverse events, vital signs, electrocardiograms, and laboratory parameters. Pharmacokinetic endpoints included exposure of subjects to felblinac and metabolites of the drug in plasma, urine, and feces.
Results
Felblinac time to maximum plasma concentration was obtained at 0.5 h, corresponding to the end of the infusion. Maximum plasma concentration and area under the curve increased in a dose-dependent manner for felblinac and its metabolite, showing linear pharmacokinetic characteristics at single and multiple doses. After intravenous infusions of multiple doses three times (30 min each time) per day, the accumulation ratio of felblinac and its metabolite based on the area under the curve had a range of 1.34–1.45 and 1.60–1.87, respectively, across cohorts. After administration of the fourth dose, the plasma concentration of both felblinac and its metabolites was maintained at a steady state. Felbinac trometamol was well tolerated. Neither treatment-emergent adverse event frequency nor severity increased with increasing felbinac trometamol dose.
Conclusions
Felbinac trometamol was well tolerated in our study. Based on the dose range in this study, 94.25 mg is the recommended target dose for a phase II study.
Clinical Trial Registration
CTR20170496 and CTR20180896. The dates of registration are 2017-06-19 and 2018-07-02 (https://www.chinadrugtrials.org.cn/).

Similar content being viewed by others
References
Ingrasciotta Y, Sultana J, Giorgianni F, Menditto E, Scuteri A, Tari M, et al. Analgesic drug use in elderly persons: a population-based study in Southern Italy. PLoS ONE. 2019;14(9):e0222836. https://doi.org/10.1371/journal.pone.0222836.
Schechter BA. Use of topical bromfenac for treating ocular pain and inflammation beyond cataract surgery: a review of published studies. Clin Ophthalmol. 2019;13:1439–60.
Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55(2):108–33.
Guo CG, Leung WK. Potential strategies in the prevention of nonsteroidal anti-inflammatory drugs-associated adverse effects in the lower gastrointestinal tract. Gut Liver. 2020;14(2):179–89. https://doi.org/10.5009/gnl19201.
Baraf HS, Fuentealba C, Greenwald M, et al. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the etoricoxib versus diclofenac sodium gastrointestinal tolerability and effectiveness (EDGE) trial. J Rheumatol. 2007;34:408–20.
Verma P, Prajapati SK, Yadav R, Senyschyn D, Shea PR, Trevaskis NL. Single intravenous dose of novel flurbiprofen-loaded proniosome formulations provides prolonged systemic exposure and anti-inflammatory effect. Mol Pharm. 2016;13(11):3688–99.
Motov S, Yasavolian M, Likourezos A, Pushkar I, Hossain R, Drapkin J, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177–84.
Wang RD, Sheng XR, Guan WX, Wang M, Peng C, Yang YY, et al. Flurbiprofen axetil for postoperative analgesia in upper abdominal surgery: a randomized, parallel controlled, double-blind, multicenter clinical study. Surg Today. 2020. https://doi.org/10.1007/s00595-019-01951-1.
Wladis EJ, Lee KW, De A. Intravenous ketorolac reduces pain score and opioid requirement in orbital surgery. Ophthalmic Plast Reconstr Surg. 2020;36(2):132–4. https://doi.org/10.1097/IOP.0000000000001484.
Brogden RN, Heel RC, Speight TM, Avery GS. Fenbufen: a review of its phar-macological properties and therapeutic use in rheumatic diseases and acute pain. Drugs. 1981;21:1–22.
Shinkai N, Korenaga K, Takizawa H, Mizu H, Yamauchi H. Percutaneous penetration of felbinac after application of transdermal patches: relationship with pharmacological effects in rats. J Pharm Pharmacol. 2008;60:71–6.
Zhang C, Cui X, Yang Y, Gao F, Sun Y, Gu J, et al. Pharmacokinetics of felbinac after intravenous administration of felbinac trometamol in rats. Xenobiotica. 2011;41(4):340–8.
Cui Y, Lin X, Guan TT, Zhang Y, Tang X. A new rapid ultra-performance liquid chromatography method for the pharmacokinetic and bioavailability study of diclofenac sodium aqueous injection and lipid microsphere injection in rats. Biomed Chromatogr. 2010;24:406–12.
Wu H, Chen Z, Sun G, Gu K, Pan Y, Hao J, et al. Intravenous flurbiprofen axetil can increase analgesic effect in refractory cancer pain. J Exp Clin Cancer Res. 2009;28:33.
Flurbiprofen. LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
World Medical Association Declaration of Helsinki. Recommendations guiding medical doctors in biomedical research involving human subjects. Ferney-Voltaire, France, 1989. Available from: https://fda.gov/oc/health/helsinki89.html. [Accessed 17 Aug 2007].
World Health Organization. Handbook for good clinical research practice (GCP). Available from: https://www.who.int/medicines/. [Accessed 20 May 2020].
Scheiman JM. NSAID-induced gastrointestinal injury: a focused update for clinicians. J Clin Gastroenterol. 2016;50(1):5–10.
Yeomans ND, Graham DY, Husni ME, Solomon DH, Stevens T, et al. Randomised clinical trial: gastrointestinal events in arthritis patients treated with celecoxib, ibuprofen or naproxen in the PRECISION trial. Aliment Pharmacol Ther. 2018;47(11):1453–63.
Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A. Flurbiprofen therapy and CYP2C9 genotype. National Center for Biotechnology Information; 2012.
Han MH, Nam JH, Noh E, Lee EK. Gastrointestinal risk of non-steroidal anti-inflammatory drugs and gastroprotective agents used in the treatment of osteoarthritis in elderly patients: a nationwide retrospective cohort study. Int J Clin Pharmacol Ther. 2019;57(11):531–41.
Fernandes E, Soares TB, Gonçalves H, Bernstorff S. A molecular biophysical approach to diclofenac topical gastrointestinal damage. Int J Mol Sci. 2018;19(11):3411.
US FDA. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. [Accessed 2 Mar 2008].
Takayama K, Hirose A, Suda I, Miyazaki A, Oguchi M, Onotogi M, et al. Comparison of the anti-inflammatory and analgesic effects in rats of diclofenac-sodium, felbinac and indomethacin patches. Int J Biomed Sci. 2011;7(3):222–9.
Acknowledgements
The authors thank the volunteers enrolled in this trial, as well as the staff who contributed to this trial. The authors also express their gratitude to Mr. Jianfei Li (Clinical Project Manager at Shijiazhuang Yiling Pharmaceutical Co., Ltd.) for his contributions to the management of the trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was financially sponsored by the following programs: National Major Scientific and Technological Special Project for Significant New Drug Development during the Thirteenth Five-Year Plan Period of China (Project: 2017ZX09304004, 2017ZX09101001-002-004), the National Natural Science Foundation of China (Project: 81602897), the program for JLU Science and Technology Innovative Research Team (2017TD-08), and the Fundamental Research Funds for the Central Universities.
Conflict of Interest
Min Wu, Cuiyun Li, Hong Zhang, Jixuan Sun, Xiaoxue Zhu, Xiaojiao Li, Xuedong Gao, Wei Wang, Yanhua Ding and Li Liu have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The clinical study protocol was approved by the Ethics Committee at the Jilin University First Affiliated Hospital-Clinical Research Institute in Changchun City, Jilin Province, China. Clinical procedures were conducted in the Phase I Clinical Trial Unit of the First Hospital of Jilin University. The studies (Registration Nos.: CTR20170496 and CTR20180896, https://www.chinadrugtrials.org.cn/) were conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice.
Informed consent
Written informed consent was obtained from each participant before the study commenced.
Rights and permissions
About this article
Cite this article
Wu, M., Li, C., Zhang, H. et al. Pharmacokinetics, Safety, and Tolerability of Intravenous Felbinac Trometamol in Healthy Chinese Volunteers: A First-in-Human Single- and Multiple-Dose Escalation Phase I Study with a Randomized, Double-Blind, Placebo-Controlled Design. CNS Drugs 34, 867–877 (2020). https://doi.org/10.1007/s40263-020-00739-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00739-z