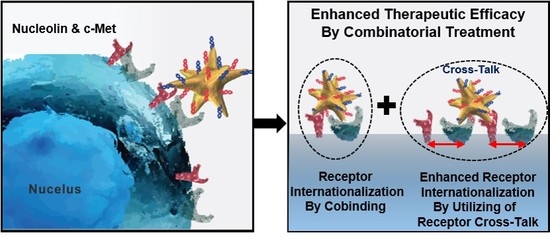
1. Introduction
The treatment of many malignant diseases through the use of combinatorial drug therapies has recently attracted the attention of physicians and scientists [
1,
2,
3,
4]. Because minimizing overlapping toxicity and drug resistance is critically important for their clinical success, specific target molecules at the appropriate concentrations should be used [
1,
4].
Nucleolin (NCL) on the cell surface is a novel cancer target molecule for tumor therapy, due to its abundance on the surface and prominence in various cancer cells, including gastric, breast, lung, and prostate cancers [
5,
6,
7]. In this context, anti-cancer drugs, such as immunogens, peptides, and aptamers (apts), have been intensively investigated for anti-NCL therapy [
8,
9,
10]. Among these molecules, AS1411 is a novel nucleolin-targeted DNA aptamer and a representative anti-NCL agent that induces apoptosis through the destabilization of bcl2 mRNA by significantly inhibiting DNA synthesis and promoting the accumulation of cells in the S phase [
11,
12]. Although promising, AS1411 faces some fundamental limitations, including a short circulatory half-life, unexpected immune responses, and insufficient anti-cancer outcomes. These drawbacks have been mitigated using a variety of methods, such as by modifying the aptamer, conjugating the aptamer with a nanocarrier, or utilizing combinatorial treatment with other anti-cancer reagents [
7].
For combinatorial treatment, the antagonism of reagents should be taken into account [
13]. Many studies have reported that receptor tyrosine kinases (RTKs) are correlated with AS1411 activity. Epithelial growth factor receptor (EGFR) decreases the ability of AS1411 to stimulate macropinocytosis, whereas the inhibition of other RTKs (HER2, MET, PDGFR, IGFR-1) has little or no effect on the cellular uptake stimulated by AS1411 [
12]. c-Met is an RTK member and a cell surface molecule for hepatocyte growth factor (HGF). It is considered an ideal target due to the fact that it is a key regulator of tumor development, including cancer cell invasion, growth, and vascularization [
13]. For this reason, the market for anti-c-Met drugs, including anti-c-Met antibodies and anti-c-Met aptamers, has been boosted by the development of anti-nucleolin reagents [
13,
14,
15].
When dual-targeting, nanoconstructs are actively used as a component of the delivery system [
16,
17]. Since the cellular capacity for the uptake of drugs and nanoconstructs is often limited [
18,
19], sophisticated systems are necessary to produce a synergistic effect [
20]. The shape of the nanoconstructs, the drug composition, and the combination of targeting molecules should be carefully considered without compromising the fidelity of the dual treatment during strategic planning and implementation [
20,
21].
In this study, we propose a novel strategy through the use of a nucleolin-targeting aptamer (AS1411, N) and c-MET-targeting aptamer (c-MET apt, C) in a synergistic manner as an effective combinatorial treatment for cancer. To improve the delivery efficacy of two aptamers into gastric cancer cells (MKN-45), we synthesized star-shaped gold nanostructured (AuNS) carriers. Bi-functional AuNS or c-MET-nucleolin AuNS (AuNS-CN) alternately bind to c-Met and nucleolin on the cellular surface. More importantly, we are the first to find that the function of both nucleolin and c-MET was simultaneously depressed after the binding of AuNS-CN to a target receptor (NCL or c-MET) through interaction between NCL and c-MET. These reactions resulted in the enhancement of the anti-cancer effect in gastric cancer. The established nanocomplex increased the therapeutic efficacy of treatment by about two-fold compared with the single apt-functionalized AuNS. Using gene set enrichment analysis (GSEA) and the Molecular Signatures Database (MSigDB), AuNS-CN was found to induce exclusive enrichment patterns of gene signatures relevant to the cell cycle, representing potential unique, indirect targets for combinatorial treatment. Finally, AuNS-CN was found to induce tumor regression in vivo, while the concurrent deployment of c-Met-AuNS (AuNS-C) and nucleolin-AuNS (AuNS-N) had no significant anti-tumor efficacy. Our findings indicate that bi-functional AuNS-CN plays an important role in suppressing and eliminating the interaction between c-Met and nucleolin, ultimately resulting in cell death.
2. Materials and Methods
2.1. Materials
Roswell Park Memorial Institute (RPMI)-1640 (Invitrogen, Carlsbad, CA, USA), fetal bovine serum (FBS) (Invitrogen), 1% antibiotic-antimycotic (AA) solution (Invitrogen), Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Invitrogen), 1,4-dithiothreitol (DTT) (Sigma-Aldrich, St. Louise, MO, USA), radioimmunoprecipitation assay (RIPA) buffer (89900; Pierce), bicinchoninic acid assay (23225; ThermoFisher, Waltham, MA, USA), sample buffer (125 mM Tris pH 6.8, 4% sodium dodecyl sulfate (SDS), 10% glycerol, 0.006% bromophenol blue, and 1.8% mercaptoethanol), precast protein gel (4561094; Bio-Rad, Hercules, CA, USA), primary antibodies, anti-c-Met (ab51067; Abcam, Cambridge, UK; 4560; Cell Signaling, Massachusetts, USA), anti-nucleolin (ab22578/ ab13541; Abcam), anti-HER2 (SC-08; Santa Cruz, TX, USA), anti-GAPDH (SC-47724; Santa Cruz), MTS assay solution (G3585; Promega, WI, USA), μ-Slide 8-well (ibid; München, Germany), Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Labs), and Matrigel (BD Biosciences, NJ, USA) were used.
2.2. Cell Culture
All cell lines were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). MKN-45 and SKBR-3 cell lines were grown in Roswell Park Memorial Institute (RPMI)-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen) and 1% antibiotic-antimycotic (AA) solution (Invitrogen). A549 cells were grown in DMEM (Gibco, Invitrogen) supplemented with 10% FBS and 1% AA. All cell lines were grown at 37 °C and 5% CO2.
2.3. Aptamer-Functionalized AuNS Synthesis
Anti-nucleolin, anti-HER2, and anti-c-Met aptamers with disulfide modification at the 5′-end were purchased from IDT DNA Inc. (IDT, Coralville, IA, USA). The sequences were as follows: anti-nucleolin, 5′-(C6-S-S-C6)-TTTGGTGGTGGTGGTTGTGGTGGTGGTG-3′; anti-HER2, 5′-(C6-S-S-C6)-GCAGCGGTGTGGGGGCAGCGGTGTGGGGGCAGCGGTGTGGGG-3′; anti-c-Met, 5′-(C6-S-S-C6)-ATCAGGCTGGATGGTAGCTCGGTCGGGGTGGGTGGGTTGGCAAGTCTGAT-3′. High-performance liquid chromatography (HPLC)-purified aptamers were dissolved in Millipore water (18.2 MΩ cm) to obtain 100 µM solutions. Disulfide bonds were cleaved by adding 100 μL of 100 mM 1,4-dithiothreitol (DTT (Sigma-Aldrich, St. Louis, MN, USA) to 100 μL of aptamer solution. After 1 h, DTT was removed, and the thiolated aptamer was isolated using a Nap-5 column. The concentration of thiolated DNA was calculated by measuring the absorbance at 260 nm. The aptamer solution was added to 0.3 nM AuNS (final concentration ratio of DNA:AuNS = 1600:1) in 25 mM citrate buffer (pH 3) and incubated overnight to allow for the formation of nanoconstructs (anti-nucleolin-AuNS, anti-HER2-AuNS, and anti-c-Met-AuNS). To prepare the bifunctional nanoconstruct, two types of reduced aptamers were mixed at a 1:1 ratio (v/v). The mixture was then added to the AuNS solution.
2.4. Quantification of Aptamer-Loading Capacity of AuNS
Cy3-labeled nucleolin aptamers 5′-(C6-S-S-C6)-Cy3-TTTGGTGGTGGTGGTTGTGGTGGTGGTG-3′ and Cy5-labeled c-Met aptamers 5′-(C6-S-S-C6)-Cy5-ATCAGGCTGGATGGTAGCTCGGTCGGGGTGGGTGGGTTGGCAAGTCTGAT-3′ were used to estimate the number of aptamers on each particle. Attachment of Cy3-nucleolin and Cy5-c-Met to AuNS was performed as previously described. A total of 500 μL of fluorophore-labeled aptamer AuNS were centrifuged at 13,500 rpm for 11 min. The supernatant was removed, and the nanoconstructs were suspended in 1 mL of 50 mM HEPES buffer. This process was repeated twice to eliminate unbound aptamers. Fluorescence-labeled nanoconstruct pellets were treated with 50 μL of 25 mM potassium cyanide (KCN) overnight to dissolve the Au core of the nanoconstructs and release the Cy3 or Cy5 aptamers. The fluorescence intensity of the KCN solution was measured using a NanoDrop spectrophotometer, and the concentration of the aptamer was determined based on the intensity of the standard curve signal.
2.5. Western blot Analysis
The cells (5 × 105 cells/mL) were cultured in a 6 well plate in complete medium for 24 h. After treating with the nanoconstructs, the cells were collected and transferred to microcentrifuge tubes. For cell lysis, radioimmunoprecipitation assay (RIPA) buffer (89900, Pierce) was added to the cells and incubated for 30 min on ice. The protein amount was estimated using a bicinchoninic acid assay (23225; ThermoFisher, Waltham, MA, USA). Briefly, an equal volume of sample buffer (125 mM Tris pH 6.8, 4% sodium dodecyl sulfate (SDS), 10% glycerol, 0.006% bromophenol blue, and 1.8% mercaptoethanol) was added to all samples, and the resulting solution was boiled for 5 min. A total of 15 μg of total protein from cells were loaded into each well of a precast protein gel (4561094; Bio-Rad, Hercules, CA, USA). After electrophoresis at 120 V for 60 min, the proteins were transferred from the gel onto a polyvinylidene fluoride (PVDF) membrane at 1 A constant current for 1 h in transfer buffer (Thermo Fisher). The blot from the transfer apparatus was removed and immediately placed into the blocking buffer (5% non-fat dry milk, 10 mM Tris pH 7.5, 100 mM sodium chloride (NaCl), and 0.1% Tween-20). After blocking for 1 h at room temperature, the membrane was incubated with primary antibodies against c-Met (ab51067; Abcam, Cambridge, UK; 4560; Cell Signaling, Beverly, MA, USA), anti-nucleolin (ab22578/ab13541; Abcam), anti-HER2 (SC-08; Santa Cruz, TX, USA), and anti-GAPDH (SC-47724; Santa Cruz) overnight at 4 °C. After incubation with the primary antibody solution, the membrane was washed twice (10 mM Tris pH 7.5, 100 mM NaCl, and 0.1% Tween-20) and incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (secondary antibody) diluted in 5% non-fat dry milk solution at room temperature. After 1 h of incubation, the antibody solution was removed, and the membrane was washed three times with a washing buffer (10 mM Tris pH 7.5, 100 mM NaCl, and 0.1% Tween-20). The protein bands were then detected with an enhanced chemiluminescence substrate (Atto, Tokyo, Japan). The amount of each protein in the blots was determined by counting the total number of pixels in each band (integrated density value) using ImageJ.
2.6. Analysis of Cell Viability by the MTS Assay
After incubating the cells (1 × 104 cells/mL) with the nanoconstructs in a 96 well plate, the cell viability was measured using MTS assay solution (G3585; Promega, WI, USA). The absorbance of the reaction solution was measured at a wavelength of 490 nm using a 96 well plate reader (Infinite 200 Pro; Tecan, Männedorf, Switzerland).
2.7. Immunofluorescence Staining
MKN-45 cells were seeded on an 8 well μ-Slide (ibid, München, Germany) at a density of 2 × 104 cells/well for 24 h. The cells were treated with AuNS aptamers for 8 or 18 h at 37 °C, followed by fixation with 4% paraformaldehyde in PBS for 10 min. The cells were permeabilized with 0.1% Triton X-100 in PBS, blocked with 5% bovine serum albumin (BSA) in PBS at room temperature, and incubated with primary antibodies at 4 °C overnight. The cells were incubated with secondary antibodies at room temperature for 1 h and mounted with Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Labs). Images were obtained using a confocal laser scanning microscope (LSM700; Carl Zeiss, Oberkochen, Germany). Primary antibodies for c-Met (NBP2-44306; Novous, Vancouver, Canada) and nucleolin (ab22758; Abcam) were used.
2.8. Immunoprecipitation
Anti-nucleolin antibody was immobilized onto the surface of magnetic particles via carboxylic acid and amine coupling reaction using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) reagent. These antibody-magnetic particles were incubated with MKN-45 cell lysate overnight at 4 °C. To determine the nonspecific protein binding of particles, bare magnetic particles (carboxylate) were incubated with the lysate. After incubation, the particles were washed twice with 1× PBS. For Western blotting, the proteins captured with anti-nucleolin magnetic particles were denatured at 95 °C using loading buffer containing DTT. After gel electrophoresis, each target protein was detected using specific antibodies. Cell lysates at the same concentration were used as a control.
2.9. Gene Analysis
MKN-45 cells were treated with AuNS aptamers for 24 h at 37 °C. After incubation, the cells were washed with 1× PBS and collected using trypsin-ethylenediaminetetraacetic acid (EDTA). Total RNA was extracted and used to synthesize cDNA. The samples were then applied to an Agilent 4 × 44 K Whole Human Genome microarray (Agilent Technologies, Amstelveen, Netherlands), hybridized, and washed according to the manufacturer’s instructions. The chips were scanned using an Agilent Microarray Scanner (Agilent Technologies, Amstelveen, The Netherlands).
2.10. MKN-45 Tumor Xenografts
All animal maintenance and in vivo experiments were conducted according to the regulations of the Institutional Animal Care and Institutional Animal Care and Use Committee of the Korea Institute of Science and Technology (project identification code: 2018-090, date: 23 November 2018). MKN-45 xenografts were generated by subcutaneously injecting 2 × 106 MKN-45 cells into the dorsal flanks of female Balb/C nude mice. The experiments were initiated once the diameter of the resulting tumors was approximately 4 mm, approximately one week after injection. To evaluate the in vivo anti-cancer efficacy, 250 nM of AuNS aptamers were intratumorally injected three times at 1 week intervals (PBS for the control group). The individual tumor volume (V) was monitored every week for 4 weeks and determined using the following formula: V = (length × width2). At 7 days after treatment, the mice were sacrificed, and their tumors were dissected for further investigation.
2.11. Hematoxylin and Eosin Staining
For histological observations, the collected xenograft tumors were fixed overnight in 10% formalin (Sigma-Aldrich), embedded in paraffin, and sliced into 6 μm sections using a microtome (Leica, Wetzlar, Germany). The sections were deparaffinized in xylene and rehydrated using graded ethanol. The tumor sections were stained with H&E using standard protocols and observed under a light microscope (Olympus, Tokyo, Japan).
2.12. TUNEL Assay
To evaluate apoptosis, the sections of tumors embedded in paraffin were stained with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) using a commercially available TUNEL assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Briefly, after routine deparaffinization and rehydration, the tumor sections were fixed with 4% formaldehyde in PBS for 15 min and washed with PBS. Proteinase K (20 µg/mL) in PBS was treated for 10 min at room temperature (RT) to digest the sections. After washing in PBS, four percent formaldehyde was applied to the sections for 5 min at RT to repeat the fixation, and the sections were immediately washed with PBS. The sections were treated with equilibration buffer for 10 min at RT before incubating with terminal deoxynucleotidyl transferase (TdT) reaction mix for 60 min at 37 °C in a humidity chamber. The reaction was terminated using 2× saline-sodium citrate stop buffer for 15 min at RT. After washing with PBS, the sections were mounted using Vectashield® with DAPI (Vector Labs). For the quantitative analysis of the TUNEL assay, we adopted the apoptotic index, which is determined by the percentage of apoptotic cells counted in five randomly chosen fields at 200× magnification (n = 4 in each group).
2.13. Immunohistological Evaluation
To estimate angiogenesis and cell proliferation in xenograft tumors, immunofluorescence staining was performed using the rabbit polyclonal anti-von Willebrand factor (vWF at 1:100) (ab6994; Abcam, Cambridge, UK) and mouse monoclonal anti-Ki 67 (1:100) (ab238020; Abcam) as the primary antibodies. The vWF- and Ki 67-positive areas in five random fields (200× magnification) were quantified using ImageJ software (n = 4 in each group). The positive areas were quantified as the ratio of the vWF- or Ki 67-positive area to the total cell expression area.
2.14. Statistical Analysis
Student’s t-test was used to determine the statistical significance (p-value) for each experiment. All error bars represent the mean ± standard deviation (SD). Data were considered statistically significant with p-values < 0.01 (*), p < 0.001 (**), and p < 0.0001 (***).
4. Conclusions
The overexpression of cell surface receptors is a key factor in cancer progression and metastasis. Moreover, receptor crosstalk has significant implications for drug resistance. c-Met is one of the most valid therapeutic targets against different types of cancer, while nucleolin is also a well-known and promising target for cancer treatment due to its abundance in cancer cells. Despite the significance of targets in cancer therapy, there has been no previous evidence demonstrating an interaction between c-Met and nucleolin via a nanoconstruct. In this study, we demonstrated that c-Met and nucleolin interact with each other at the molecular level and are thus ideal combinatorial targeting partners for use in cancer treatment. Nucleolin is a member of the HGF receptor family; therefore, it responds to HGF stimulation in the absence of c-Met [
31]. Based on our finding that nucleolin binds to c-Met, it is possible that nucleolin acts as a co-receptor for the c-Met signaling pathway.
It has been previously reported that limited cellular uptake of nanoconstructs affects the efficacy of drug delivery [
18]. These studies have also shown that the expression levels of the receptors can be a limiting factor for the cellular capacity of the receptor-mediated uptake of nanoconstructs. Therefore, a balanced and optimum combination of targeting receptors is needed prior to drug delivery. In our study, sequential treatment with nanoconstructs resulted in relatively lower therapeutic efficacy compared to simultaneous drug treatments. Since the uptake machinery of cells is limited, it is possible that the interaction between the nanoconstruct and the cell gradually diminished with changes in cellular metabolism. This highlights the need for appropriate combinatorial receptor co-targeting for an effective drug delivery with an enhanced therapeutic efficacy.
Importantly, the aptamers on the surface of AuNS are more stable under physiological conditions than free aptamer molecules. The negatively charged aptamer AuNS recruits salt ions, resulting in a high local salt concentration around the particle surface [
32]. As a result, the nuclease enzymatic activity decreases at the AuNS surface, which contributes to an improved aptamer stability in the body. The immobilization of aptamers onto the surface of AuNS at a high density was found to improve the anti-cancer effect, as well as increase the targeting efficacy, simultaneously, for the application of therapeutic aptamers in vivo.
In summary, this study aimed to establish a bi-functional AuNS-CN nanoconstruct-based cancer therapy using the simultaneous c-Met and nucleolin targeting approach. We demonstrated that AuNS-CN synergistically enhanced the anti-cancer therapeutic efficacy without any noticeable compromises compared to treatment with the AuNS single molecule. The enhanced therapeutic efficacy appeared to originate from the proximity between the c-Met and nucleolin targeted by AuNS-CN. This enhanced performance of the AuNS-CN therapy was confirmed through additional experiments, wherein the enrichment of the nucleolin component of the AuNS-CN on the membrane following AuNS-CN binding to c-Met resulted in a better inhibition of nucleolin activity, and vice versa. Transcriptome analysis showed that AuNS-CN exhibited exclusive enrichment patterns of gene signatures related to the cell cycle. To the best of our knowledge, this synergistic AuNS-CN cancer therapy is the first demonstration of the inhibition of the interaction between c-Met and nucleolin. However, despite the novel finding that c-Met and nucleolin are synergetic partners and have potential applications as a cancer treatment, further study is needed to elucidate the interaction between the two receptors. We anticipate optimized combinatorial treatments targeting receptor interaction to promote the development of new solutions with which to overcome drug resistance and enhance the therapeutic efficacy of treatments against aggressive diseases.