Abstract
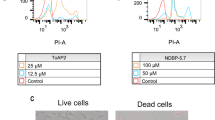
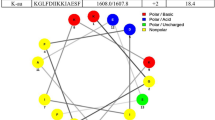
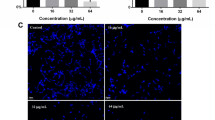
Increasing resistance and changes in the spectrum of Candida infections have generated considerable interest in the development of new antifungal molecules. The use of antimicrobial peptides (AMPs) appears to be a promising approach. Frog skin AMPs (such as dermaseptins) have shown antimicrobial activity against several pathogens. In this study, we aimed to test the antimicrobial efficacy of dermaseptin S4 (DS4) against C. albicans. We determined the minimal inhibitory concentration (MIC) of DS4, and investigated the effects of the DS4 at low concentrations on human primary gingival fibroblasts. Additionally, we evaluated the effect of DS4 on C. albicans growth, form changes, and biofilm formation, as well as the expression of certain virulent genes. Our data show that DS4 completely inhibits C. albicans growth at a concentration of 32 μg/mL referring to the MIC of DS4. It should be noted that even with low concentrations (below 16 μg/mL), DS4 still have significant growth reduction of C. albicans, but were not toxic to human gingival fibroblasts. DS4 inhibited the transition from yeast to hyphae, and decreased the biofilm formation by reducing the biofilm mass weight. Surface morphological changes in the yeast cell membrane were observed following exposure to DS4. The gene expression analyses revealed that DS4 significantly decreased the expression of EAP1 and HWP1 genes. Overall results suggest the potential use of DS4 as an antifungal therapy to prevent C. albicans pathogenesis.







Similar content being viewed by others
References
Enoch DA, Yang H, Aliyu SH, Micallef C (2017) The changing epidemiology of invasive fungal infections. Methods Mol Biol 1508:17–65. https://doi.org/10.1007/978-1-4939-6515-1_2
Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN (2019) Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997-2016. Open Forum Infect Dis 6(Suppl 1):S79–S94. https://doi.org/10.1093/ofid/ofy358
Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. N Engl J Med 373(15):1445–1456. https://doi.org/10.1056/NEJMra1315399
Nobile CJ, Johnson AD (2015) Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92. https://doi.org/10.1146/annurev-micro-091014-104330
Soll DR, Daniels KJ (2016) Plasticity of Candida albicans biofilms. Microbiol Mol Biol Rev 80(3):565–595. https://doi.org/10.1128/MMBR.00068-15
Tsui C, Kong EF, Jabra-Rizk MA (2016) Pathogenesis of Candida albicans biofilm. Pathog Dis 74 (4):ftw018. doi:https://doi.org/10.1093/femspd/ftw018
Dadar M, Tiwari R, Karthik K, Chakraborty S, Shahali Y, Dhama K (2018) Candida albicans - biology, molecular characterization, pathogenicity, and advances in diagnosis and control - an update. Microb Pathog 117:128–138. https://doi.org/10.1016/j.micpath.2018.02.028
Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4(2):119–128. https://doi.org/10.4161/viru.22913
Chaffin WL (2008) Candida albicans cell wall proteins. Microbiol Mol Biol Rev 72(3):495–544. https://doi.org/10.1128/MMBR.00032-07
Orsi CF, Borghi E, Colombari B, Neglia RG, Quaglino D, Ardizzoni A, Morace G, Blasi E (2014) Impact of Candida albicans hyphal wall protein 1 (HWP1) genotype on biofilm production and fungal susceptibility to microglial cells. Microb Pathog 69-70:20–27. https://doi.org/10.1016/j.micpath.2014.03.003
Li F, Palecek SP (2003) EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot Cell 2(6):1266–1273. https://doi.org/10.1128/ec.2.6.1266-1273.2003
Li F, Svarovsky MJ, Karlsson AJ, Wagner JP, Marchillo K, Oshel P, Andes D, Palecek SP (2007) Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukaryot Cell 6(6):931–939. https://doi.org/10.1128/EC.00049-07
Nobile CJ, Nett JE, Andes DR, Mitchell AP (2006) Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell 5(10):1604–1610. https://doi.org/10.1128/EC.00194-06
Robbins N, Caplan T, Cowen LE (2017) Molecular evolution of antifungal drug resistance. Annu Rev Microbiol 71:753–775. https://doi.org/10.1146/annurev-micro-030117-020345
Nett JE, Andes DR (2016) Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin N Am 30(1):51–83. https://doi.org/10.1016/j.idc.2015.10.012
Lin GY, Chen HF, Xue YP, Yeh YC, Chen CL, Liu MS, Cheng WC, Lan CY (2016) The antimicrobial peptides P-113Du and P-113Tri function against Candida albicans. Antimicrob Agents Chemother 60(10):6369–6373. https://doi.org/10.1128/AAC.00699-16
Theberge S, Semlali A, Alamri A, Leung KP, Rouabhia M (2013) C. albicans growth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol 13:246. https://doi.org/10.1186/1471-2180-13-246
Ladram A, Nicolas P (2016) Antimicrobial peptides from frog skin: biodiversity and therapeutic promises. Front Biosci (Landmark Ed) 21:1341–1371. https://doi.org/10.2741/4461
Nicolas P, El Amri C (2009) The dermaseptin superfamily: a gene-based combinatorial library of antimicrobial peptides. Biochim Biophys Acta 1788(8):1537–1550. https://doi.org/10.1016/j.bbamem.2008.09.006
Belmadani A, Semlali A, Rouabhia M (2018) Dermaseptin-S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J Appl Microbiol 125(1):72–83. https://doi.org/10.1111/jam.13745
Mor A, Nicolas P (1994) Isolation and structure of novel defensive peptides from frog skin. Eur J Biochem 219(1–2):145–154. https://doi.org/10.1111/j.1432-1033.1994.tb19924.x
Rodriguez-Tudela JL, Arendrup MC, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Denning DW, Donnelly JP, Dromer F, Fegeler W, Lass-Flörl C, Moore C, Richardson M, Sandven P, Velegraki A, Verweij P, Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing (2008) EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 14(4):398–405. https://doi.org/10.1111/j.1469-0691.2007.01935.x
Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W, AFST EUCAST (2012) EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18(7):E246–E247. https://doi.org/10.1111/j.1469-0691.2012.03880.x
Alanazi H, Park HJ, Chakir J, Semlali A, Rouabhia M (2018) Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem Toxicol 118:390–398. https://doi.org/10.1016/j.fct.2018.05.049
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Benzaid C, Belmadani A, Djeribi R, Rouabhia M (2019) The effects of Mentha x piperita essential oil on C. albicans growth, transition, biofilm formation, and the expression of secreted aspartyl proteinases genes. Antibiotics (Basel) 8(1). https://doi.org/10.3390/antibiotics8010010
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lum KY, Tay ST, Le CF, Lee VS, Sabri NH, Velayuthan RD, Hassan H, Sekaran SD (2015) Activity of novel synthetic peptides against Candida albicans. Sci Rep 5:9657. https://doi.org/10.1038/srep09657
Zhou X, Shi D, Zhong R, Ye Z, Ma C, Zhou M, Xi X, Wang L, Chen T, Kwok HF (2019) Bioevaluation of ranatuerin-2Pb from the frog skin secretion of Rana pipiens and its truncated analogues. Biomolecules 9(6). https://doi.org/10.3390/biom9060249
Conlon JM, Galadari S, Raza H, Condamine E (2008) Design of potent, non-toxic antimicrobial agents based upon the naturally occurring frog skin peptides, ascaphin-8 and peptide XT-7. Chem Biol Drug Des 72(1):58–64. https://doi.org/10.1111/j.1747-0285.2008.00671.x
Tan Y, Chen X, Ma C, Xi X, Wang L, Zhou M, Burrows JF, Kwok HF, Chen T (2018) Biological activities of cationicity-enhanced and hydrophobicity-optimized analogues of an antimicrobial peptide, dermaseptin-PS3, from the skin secretion of Phyllomedusa sauvagii. Toxins (Basel) 10(8). https://doi.org/10.3390/toxins10080320
Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q (2019) The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11(7):3919–3931
Park HJ, Salem M, Semlali A, Leung KP, Rouabhia M (2017) Antimicrobial peptide KSL-W promotes gingival fibroblast healing properties in vitro. Peptides 93:33–43. https://doi.org/10.1016/j.peptides.2017.05.003
Porat Y, Marynka K, Tam A, Steinberg D, Mor A (2006) Acyl-substituted dermaseptin S4 derivatives with improved bactericidal properties, including on oral microflora. Antimicrob Agents Chemother 50(12):4153–4160. https://doi.org/10.1128/AAC.00750-06
Lima PG, Souza PFN, Freitas CDT, Oliveira JTA, Dias LP, Neto JXS, Vasconcelos IM, Lopes JLS, Sousa DOB (2020) Anticandidal activity of synthetic peptides: mechanism of action revealed by scanning electron and fluorescence microscopies and synergism effect with nystatin. J Pept Sci:e3249. doi:https://doi.org/10.1002/psc.3249
Harris M, Mora-Montes HM, Gow NAR, Coote PJ (2009) Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface. Microbiology 155(Pt 4):1058–1070. https://doi.org/10.1099/mic.0.026120-0
Luca V, Olivi M, Di Grazia A, Palleschi C, Uccelletti D, Mangoni ML (2014) Anti-Candida activity of 1-18 fragment of the frog skin peptide esculentin-1b: in vitro and in vivo studies in a Caenorhabditis elegans infection model. Cell Mol Life Sci 71(13):2535–2546. https://doi.org/10.1007/s00018-013-1500-4
Banerjee M, Uppuluri P, Zhao XR, Carlisle PL, Vipulanandan G, Villar CC, Lopez-Ribot JL, Kadosh D (2013) Expression of UME6, a key regulator of Candida albicans hyphal development, enhances biofilm formation via Hgc1- and Sun41-dependent mechanisms. Eukaryot Cell 12(2):224–232. https://doi.org/10.1128/EC.00163-12
Niewerth M, Kunze D, Seibold M, Schaller M, Korting HC, Hube B (2003) Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. Antimicrob Agents Chemother 47(6):1805–1817. https://doi.org/10.1128/aac.47.6.1805-1817.2003
Li Y, Chang W, Zhang M, Li X, Jiao Y, Lou H (2015) Diorcinol D exerts fungicidal action against Candida albicans through cytoplasm membrane destruction and ROS accumulation. PLoS One 10(6):e0128693. https://doi.org/10.1371/journal.pone.0128693
La Rocca P, Shai Y, Sansom MS (1999) Peptide-bilayer interactions: simulations of dermaseptin B, an antimicrobial peptide. Biophys Chem 76(2):145–159. https://doi.org/10.1016/s0301-4622(98)00232-4
Amiche M, Galanth C (2011) Dermaseptins as models for the elucidation of membrane-acting helical amphipathic antimicrobial peptides. Curr Pharm Biotechnol 12(8):1184–1193. https://doi.org/10.2174/138920111796117319
Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, Clements D, Tang H, Scott RW, Tew GN, Diamond G (2007) Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother 51(11):4125–4132. https://doi.org/10.1128/AAC.00208-07
Kadosh D (2019) Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr Opin Microbiol 52:27–34. https://doi.org/10.1016/j.mib.2019.04.005
Verma-Gaur J, Traven A (2016) Post-transcriptional gene regulation in the biology and virulence of Candida albicans. Cell Microbiol 18(6):800–806. https://doi.org/10.1111/cmi.12593
Sharkey LL, McNemar MD, Saporito-Irwin SM, Sypherd PS, Fonzi WA (1999) HWP1 functions in the morphological development of Candida albicans downstream of ZhouEFG1, TUP1, and RBF1. J Bacteriol 181(17):5273–5279
Acknowledgements
The authors thank M. Amine Belmadani for his help with the extraction of the total RNA, and Dr. Abdelhabib Semlali for explanation about PCR analyses.
Funding
This work was supported by a grant (FO104103) from the “Fonds Émile-Beaulieu”, Laval University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The use of primary human gingival fibroblasts was approved by the Ethic Committee of Université Laval with approval number: 2017-014.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samot, J., Rouabhia, M. Effect of Dermaseptin S4 on C. albicans Growth and EAP1 and HWP1 Gene Expression. Probiotics & Antimicro. Prot. 13, 287–298 (2021). https://doi.org/10.1007/s12602-020-09685-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09685-0