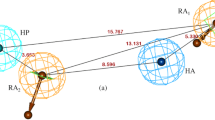
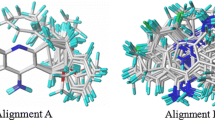
Abstract
Alzheimer’s disease (AD) is an irreversible and progressive brain disorder that slowly destroys memory and cognitive skills. The current treatment of AD mainly focused on the restoring of ACh levels through acetylcholinesterase (AChE) inhibition. Peptides are a unique class of pharmaceutical compounds that have privilege over small molecules, especially in the realm of protein–protein interactions and G protein-coupled receptor (GPCR) inhibitors. We applied a rational structure-based virtual design approach to discover new peptidic inhibitors of AChE. In this regard, conformational space in the fasciculin II (Fas) and AChE complex was evaluated utilizing MD simulation, principal component analysis and clustering to figure out possible interactions of Fas and AChE. Assessment of Fas–AChE interactions by visual evaluation and alanine scanning led to the design of 10 peptides. The highest scored peptide (p2) was selected and synthesized using SPPS. Based on Ellman's test, the inhibitory activity of p2 against AChE was 51.2 ± 8.1 µM. The kinetics study of the enzyme inhibition in accompany with molecular modeling results revealed that p2 was a mixed-type reversible inhibitor of AChE. The DNRMLRTTRY peptide was considerable inhibitor of AChE. Peptides have the merit of being big enough to inhibit PPI and GPCR class B with a wide binding site. But possible peptidic chemical space is too large to be evaluated by the classical peptide synthesis methods. In the present contribution, we introduced a rational in silico peptide design approach that led to the considerable peptidic inhibitor of AChE.







Similar content being viewed by others
References
Alvarez A, Opazo C, Alarcon R, Garrido J, Inestrosa NC (1997) Acetylcholinesterase promotes the aggregation of amyloid-beta-peptide fragments by forming a complex with the growing fibrils. J Mol Biol 272:348–361. https://doi.org/10.1006/jmbi.1997.1245
Alves CQ et al (2013) In vitro acetylcholinesterase activity of peptide derivatives isolated from two species of Leguminosae. Pharm Biol 51:936–939
Amblard M, Fehrentz JA, Martinez J, Subra G (2006) Methods and protocols of modern solid phase peptide synthesis. Mol Biotechnol 33:239–254. https://doi.org/10.1385/mb:33:3:239
Ano Y, Ayabe T, Ohya R, Kondo K, Kitaoka S, Furuyashiki T (2019) Tryptophan-tyrosine dipeptide, the core sequence of β-lactolin, improves memory by modulating the dopamine system. Nutrients 11:348
Alzheimer's Association (2018) Alzheimer's disease facts and figures. Alzheimers Dement 14:367–429
Bajda M et al (2020) Search for new multi-target compounds against Alzheimer’s disease among histamine H3 receptor ligands. Eur J Med Chem 185:111785
Bechara C, Sagan S (2013) Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett 587:1693–1702
Bolognesi ML, Cavalli A (2016) Multitarget drug discovery and polypharmacology. ChemMedChem 11:1190–1192
Bourne Y, Taylor P, Marchot P (1995) Acetylcholinesterase inhibition by fasciculin: crystal structure of the complex. Cell 83:503–512
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:014101
Craig LA, Hong NS, McDonald RJ (2011) Revisiting the cholinergic hypothesis in the development of Alzheimer's disease. Neurosci Biobehav Rev 35:1397–1409
Cummings JL (2004) Alzheimer’s disease. N Engl J Med 351:56–67
Dastan D, Validi S, Ebadi A (2020) Kamonolol acetate from Ferula pseudalliacea as AChE inhibitor: in vitro and in silico studies. Struct Chem. https://doi.org/10.1007/s11224-019-01473-z
De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC (2001a) A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 40:10447–10457. https://doi.org/10.1021/bi0101392
De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC (2001b) A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry 40:10447–10457
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Falkenstein RJ, Pena C (1999) Interaction of synthetic peptides from fasciculin with acetylcholinesterase. J Protein Chem 18:233–238
Falkenstein RJ, Peña C (1997) Synthetic peptides derived from the central loop of fasciculin: structural analysis and evaluation as inhibitors of acetylcholinesterase. Biochim Biophys Acta 1340:143–151
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20:122–128
Hampel H et al (2019) Revisiting the cholinergic hypothesis in Alzheimer’s disease: emerging evidence from translational and clinical research. J Prev Alzheimers Dis 6:2–15
Hampel H et al (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141:1917–1933
Hess B, Kutzner C, Van Der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Inestrosa NC et al (1996) Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron 16:881–891. https://doi.org/10.1016/s0896-6273(00)80108-7
Kortemme T, Baker D (2002) A simple physical model for binding energy hot spots in protein–protein complexes. Proc Natl Acad Sci 99:14116–14121
Laskowski RA, Jabłońska J, Pravda L, Vařeková RS, Thornton JM (2018) PDBsum: structural summaries of PDB entries. Protein Sci 27:129–134
Mondal P, Gupta V, Das G, Pradhan K, Khan J, Gharai PK, Ghosh S (2018) Peptide-based acetylcholinesterase inhibitor crosses the blood-brain barrier and promotes neuroprotection. ACS Chem Neurosci 9:2838–2848
Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L, Renard P-Y (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer's drugs targeting acetyl-and butyryl-cholinesterase. Biochem J 453:393–399
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Parthasarathy A, Anandamma SK, Kalesh KA (2019) The medicinal chemistry of therapeutic peptides: recent developments in synthesis and design optimizations. Curr Med Chem 26(13):2330–2355
Radić Z, Duran R, Vellom DC, Li Y, Cervenansky C, Taylor P (1994) Site of fasciculin interaction with acetylcholinesterase. J Biol Chem 269:11233–11239
Razzaghi-Asl N, Ebadi A (2020) In silico design of peptide inhibitors of tubulin: amyloid-β as a lead compound. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1764391
Saxena A, Saini R (2018) The structural hybrids of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease: a review. J Alzheimers Neurodegener Dis 4:015
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8:595–608
Van Zundert G et al (2016) The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol 428:720–725
Waqar M, Batool S (2015) In silico analysis of binding of neurotoxic venom ligands with acetylcholinesterase for therapeutic use in treatment of Alzheimer's disease. J Theor Biol 372:107–117. https://doi.org/10.1016/j.jtbi.2015.02.028
Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA (2012) The natural history of cognitive decline in Alzheimer's disease. Psychol Aging 27:1008
Yu Z et al (2018) Anti-Alzheimers activity and molecular mechanism of albumin-derived peptides against AChE and BChE. Food Funct 9:1173–1178
Zare-Zardini H, Tolueinia B, Hashemi A, Ebrahimi L, Fesahat F (2013) Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am J Alzheimers Dis Other Demen 28:702–709. https://doi.org/10.1177/1533317513500839
Acknowledgements
We would like to thank the Research and Technology Vice-chancellor of Hamadan University of Medical Sciences for financial support. This research was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9604132260).
Funding
Hamadan University of Medical Sciences, Grant Number: 9604132260.
Author information
Authors and Affiliations
Contributions
AE: experimental design, molecular modeling and SPPS25 KF: molecular modeling and SPPS26 DD: experimental design, HPLC and ESI–Ms characterization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dastan, D., Fasihi, K. & Ebadi, A. From Venom to AChE Inhibitor: Design, Molecular Modeling, and Synthesis of a Peptidic Inhibitor of AChE. Int J Pept Res Ther 27, 463–474 (2021). https://doi.org/10.1007/s10989-020-10103-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10103-w