Abstract
Large wood is a key component of river channels that affects numerous hydrological, physical and geomorphological processes. It promotes a diversity of benthic habitats in-channel and has shown to support more abundant and diverse benthic macroinvertebrate assemblages in previous ecological studies. However, the effects of large wood on the structural and functional diversities of hyporheic invertebrates are less well studied, and simultaneous examination of these diversity metrics on hyporheic and benthic compartments of the stream bed has not been conducted previously. Therefore, this study investigates the taxonomic and functional diversities of hyporheic and benthic invertebrate assemblages around natural accumulations of large wood in a British lowland river. Taxonomic and functional diversities were partitioned (into alpha, beta, and gamma diversities) and examined in reaches with and without large wood (control). We found that functional diversity is often decoupled from taxonomic diversity, demonstrating a functional redundancy of the macroinvertebrate assemblage for both hyporheic and benthic zones. Moreover, the highest functional variability at alpha-scale was observed in large wood habitats, which suggests that taxonomic diversity is enhanced by the small-scale environmental heterogeneity around large wood. To this end, this study contributes empirical evidence of functional and structural responses of invertebrates to large wood accumulation. Such information could be used to better understand the ecological implications of restoration works in lowland rivers and guide more effective management strategies.
Similar content being viewed by others
Introduction
Large wood (LW) has a profound impact on fluvial processes and ecosystems (Grabowski et al. 2019). LW drives river hydrological, physical and ecological processes, as a result of its interactions with water, sediment and biological communities (Wohl 2013). Previous ecological studies of benthic macroinvertebrate assemblages have focused on taxonomic diversity (TD) and found that LW promotes alpha and beta diversities through its effects on habitat complexity (Thompson et al. 2018), but evidence of the ecological effects of LW on the hyporheic zone (HZ) and on functional diversity (FD) have received less attention (Magliozzi et al. 2019).
Large wood is delivered naturally to rivers by upland and riparian forests, through landslides and successional or disturbance pulse processes (i.e. floods, fires, windfall, erosion) from adjacent hillslopes (Boyer et al. 2003). It is defined as living or dead wood in simple or complex structures, in which individual pieces are > 1 m length and > 10 cm diameter (Thevenet et al. 1998; Wohl et al. 2010). In the channel, LW interacts with river flows and sediment transport to influence sediment sorting and deposition (Gurnell et al. 2005). It generates a mosaic of benthic habitat patches, where spatially-varying flow, physicochemical conditions (nutrient, organic matter) and sediment grain size create the conditions for invertebrate colonization at different stages of their life cycle, e.g. for reproduction (oviposition site) and refugia from high flows and predators (Hoffmann and Hering 2000; Robertson 2000). Moreover, LW itself is a direct food source for xylophagous species, a firm substrate for biofilms and the organisms that feed upon them (i.e. scrapers), and is associated with the accumulation of organic material (Flores et al. 2011, 2013; Osei et al. 2015; Suftin et al. 2016), which provides food resources to shredders, gatherers, and filter feeders (Díez et al. 2000; De Castro-Català et al. 2015). Several studies have suggested that assemblage composition differs significantly between sites with and without wood, with higher benthic macroinvertebrate diversity observed around wood because of the increased habitat heterogeneity at these sites (Pilotto et al. 2014). In addition to the effects on taxonomic structure, LW-induced hydro-geomorphological processes act as selective forces on functional traits of invertebrates (i.e. habitat filtering; Diaz et al. 1998). Recent research has demonstrated that LW-processes favour the occurrence of specific behavioural, biological, and physiological traits within species assemblages that are dissimilar to assemblages living in sites without wood (Magliozzi et al. 2019). To this end, invertebrate assemblages that co-exist in LW might be more taxonomically and functionally diverse than assemblages in places where in-channel LW is absent. Despite extensive research on benthic macroinvertebrates, few studies have considered the impacts of LW on hyporheic assemblages (Smock et al. 1992; Wagenhoff and Olsen 2014; Magliozzi et al. 2019) or simultaneously looked at taxonomic and functional diversities of invertebrates in the benthic and hyporheic zones in relation to LW.
The hyporheic zone (HZ, Orghidan 1959) is an area of interactions between surface and ground waters in riverbeds, which is characterized by a diverse fauna (hyporheos) and a bidirectional flow of water, i.e. hyporheic exchange flow (HEF). Field and experimental studies have demonstrated the importance of wood-driven HEF in structuring the physical and the ecological compartments of river systems (Mutz and Rohde 2003; Lautz et al. 2006; Mutz et al. 2007; Fanelli and Lautz 2008). LW affects nutrient retention (Bernot and Dodds 2005), sediment hydraulic conductivity (Hess et al. 1992), oxygen concentration (Naegeli and Uehlinger 1997; Kaller and Kelso 2007) and water temperature (Sawyer et al. 2012). However, substantial gaps remain in our understanding and ability to quantify the relationship among multiple components of biodiversity for benthic and hyporheic assemblages around LW. As the HZ plays a key role in the life cycle of many benthic invertebrates (Marmonier et al. 1993; Robertson and Wood 2010; Durkota et al. 2019), a better understanding of the role of LW on the taxonomic and functional structure of the hyporheos is necessary to identify the processes controlling the functioning of the HZ.
Combining taxonomic and functional diversities approaches could deepen our understanding of biodiversity responses of invertebrate assemblages at LW sites. Functional diversity is a component of community biodiversity that represents the diversity of functional traits in a community (Mason and de Bello 2013), which has been used to infer processes governing community assembly (Mouchet et al. 2010). Coupled with taxonomic diversity, functional diversity provides information on the functional redundancy within a community (de Bello et al. 2007, 2009), that is when different species have similar functional traits. This is an important insight into the potential effects of natural and anthropogenic disturbance on the stability of the invertebrate assemblages (Gallardo et al. 2011). An increase in taxonomic diversity does not necessarily correspond to an increase in functional diversity (Petchey et al. 2007). It is currently unknown if changes in taxonomic diversity are reflected by changes in functional diversity within (alpha) and among (beta) invertebrate communities around LW. Results of recent research have suggested that functional diversity is greater in LW than no-LW sites, as hydro-geomorphological factors at LW can modulate the taxonomic-functional diversity relationship by selecting for divergent types of traits (Magliozzi et al. 2019).
Therefore, this study investigated taxonomic and functional diversities of benthic and hyporheic invertebrates at LW and control (no LW) habitats. In particular, we partitioned the diversity of the study system (gamma) into its alpha (within habitats) and beta (between habitats) components to investigate if LW affects the spatial partitioning of TD and FD of hyporheic and benthic assemblages. We hypothesized that: (i) taxonomic and functional diversities would be lower in control than LW habitats because LW creates a diverse mosaic of microhabitats that enhances the diversity of available ecological niches; (ii) for both taxonomic and functional metrics, between habitats differences would be higher in LW than control habitats because LW selects for divergent types of traits and highly diverse assemblages; and (iii) gamma diversity, and the proportion of diversity explained by the variability between habitats, would be higher in LW than in control habitats because LW provides more diverse and temporally variable resources (e.g. trapped organic matter, epixylon) than control habitats.
Material and methods
Survey design
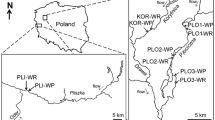
The study was conducted in the woodland Hammer stream, a major tributary of the River Rother (West Sussex, UK). The site has been used in earlier studies to investigate how river processes are affected by LW, because LW occurs naturally in the channel, and drives local HEF (Shelley et al. 2017; Magliozzi et al. 2019). Under baseflow conditions, the Hammer stream has generally slow surface water velocities, little variability in height and slope of stream water surface, average wetted width of ca. 4.5 m, and increased fining of the riverbed. HEF is primarily hydrostatically-driven, shorter in length and with a shorter residence time than upland systems (Krause et al. 2014; Shelley et al. 2017). The shorter the residence time, the smaller the impact of LW on nutrient attenuation (i.e. nitrate; Shelley et al. 2017) and oxygen availability into the streambed. Still, LW in lowland streams significantly influences total residence time by creating low velocity zones within the channel and allowing biogeochemical transformation to occur (Stofleth et al. 2008; Shelley et al. 2017; Blaen et al. 2018), and by increasing the channel blockage ratio, Froude number and sediment permeability (Mutz 2000). Therefore, geomorphological surveys were carried out to assess (i) the extent of the impounding effect of LW, (ii) the stability of the LW over time, and (iii) flow velocities upstream and downstream of LWs to calculate the distance at which the effect of LW was still significant (Table 1). The study area was separated into two sections based on substrate type: a river section upstream of an in-line static water body (Hammer Pond) with a predominantly sandy substrate, and a section downstream with predominantly gravel substrate. Four reaches were studied section, each reach containing one in-channel LW habitat and a control habitat without wood (Fig. 1). Therefore, a total of 16 habitats, eight in-channel LW and eight control habitats were sampled (Fig. 1). LW were stable during the study period and were located in reaches more than 150 m apart (i.e. > 20 times the channel width) to avoid spatial dependencies. Control habitats were selected for each reach upstream of LW (distance of ca 10 times the channel width) in an area of the riverbed without woody material (bare or without accumulated woody fragments). These habitats were unaffected by LW-induced hydrological and geomorphological processes as confirmed by cross-sections, total station, and velocity measurements. Also, this distance is sufficiently long for studies on mesohabitat scale variations in benthic (or hyporheic) invertebrates (Beisel et al. 1998, 2000).

modified from Magliozzi et al. (2019)
Study area. Invertebrate samples were collected at LW (LW1-8) and control habitats (stars symbol) in sand (upstream Hammer pond) and gravel (downstream Hammer pond) substrates. Three replicates were taken in control and around LW habitats (b) using colonization pots and a Surber net. Upper section of insert b sampling design around the wood (W) and control (c) and the colonization pot equipped with wood stakes (*) and minipiezometer (**). (b1) Pot is positioned into the river bed. (b2) Pot is extracted. Illustration
Invertebrate sampling and functional traits
Hyporheic and benthic invertebrates were sampled three times over 2016–2017 (October–November, March–April, July–August) using colonization pots and a Surber sampler (0.05 m2, mesh size = 500 µm), respectively. Three replicates were taken at each habitat (LW and control), for each sampling campaign and sampling method (3 replicates x 16 habitats x 3 sampling campaigns x 2 sampling methods = 288 samples, in total). At LW, invertebrates were sampled upstream, downstream and lateral to the structure (Fig. 1). Hyporheic samples were collected using colonization pots (Fig. 1b; 15 cm high, 8 cm diam., mesh size 1 cm2), following a procedure described in Crossman et al. (2013). Holes were excavated by shovel to ca. 25 cm deep, and colonization pots were packed with sediment in stratigraphic order and left in-situ for 6 weeks (Coleman and Hynes 1970). Each colonization pot was equipped with a tarpaulin bag with reinforced top and cable which was pulled up around the pot at the time of collection to minimize water loss during its extraction. After 6 weeks, hyporheic and benthic samples were collected on the same day. Immediately after collection, the samples were preserved in 90% ethanol. Samples were returned to the laboratory, where they were rinsed and filtered through a set of sieves. For the colonization pot samples, a 500 µm sieve was used to retain larger individuals, herein considered the hyporheic macrofauna. The rest of the sample was filtered through a 45 µm sieve and the retained invertebrates constituted the hyporheic meiofauna (Fenchel 1978). Meiofaunal samples were preserved in 100% ethanol, stained with Rose Bengal, and sorted within a few days of collection. Surber samples were collected after the colonization samples, sieved with a 500 µm sieve and the retained individuals formed the benthic macrofauna. After sorting, macrofaunal samples were preserved in 80% ethanol.
The trait dataset used in this study is based on Magliozzi et al. (2019) and consists of behavioural, biological, morphological and physiological features reflecting organismal performance and adaptations to environmental pressures. At genus or family level, the macrofauna and meiofauna functional traits were fuzzy-coded using the trait information from Tachet et al. (2010). Taxon affinities for the trait modalities were expressed as relative abundance distributions so that the sum of trait modality scores for an individual trait and taxon equals one. Some taxa were described as mean trait profiles of their potential families in the corresponding biogeographic area (i.e. Nematoda, Oligochaeta, Cyclopoida, Acari, Anomopoda, Copepoda, Ctenopoda and Ostracoda—Descloux et al. 2014).
Calculation of taxonomic and functional diversities
In this study, taxonomic and functional diversities were calculated for both benthic and hyporheic invertebrates at three spatial scales: α- (within habitats), β- (between habitats) and γ-diversity (within reaches and river sections). We computed taxonomic and functional diversities across spatial scales by using Rao’s quadratic entropy index (1982), calculated with the R function “Rao” by de Bello et al. (2010) that expresses taxonomic and functional diversities in a comparable format. The Rao index provides the most standard approach to estimate and compare measures of species dissimilarity (e.g. α, β and γ) and facets of diversity (e.g. taxonomic, phylogenetic and functional), while accounting for species relative abundances (de Bello et al. 2010; Botta-Dukat 2018). Moreover, this index provides a measure of species redundancy within and among ecological communities (de Bello et al. 2007, 2009). For calculations of the different diversity indices, the taxon abundances of the three replicates were accordingly summed per habitat, reach, campaign and/or section. The abundance data were log-transformed (x + 1) to improve data homoscedasticity.
α-diversity
α-diversity expresses the dissimilarity between two randomly chosen individuals from a sampled community. α-TD and α-FD were calculated for both benthic and hyporheic invertebrates using Rao’s index (1982) (Eq. 1):
where dij is the dissimilarity in either presence/absence or trait values, respectively for taxonomic diversity and functional diversity, between each pair of species i and j, pic and pjc are respectively the proportions of species i and species j in community c (e.g. in a given sampling unit or habitat), and s is the number of species in the community (Lepš et al. 2006; Pavoine et al. 2005). The Rao index equals the Simpson index of diversity when all the dissimilarities between species/taxa are equal to 1 (Leps et al. 2006).
For functional diversity, trait data of both benthic and hyporheic invertebrates were used to calculate a matrix of pairwise functional dissimilarities (dij) between taxa using Gower distance (Gower 1971). For this calculation, the R function “trova” was first used to account for multiple fuzzy-coded traits (de Bello et al. 2013). The function accounts for intraspecific variability in trait values by estimating the proportion of the individuals of each species that belong to each of the categories in that trait. It allows the computation of pairwise functional distances between taxa of a given assemblage from a matrix or data frame, in which taxa (“objects” in rows) are described by their affinities for a series of trait categories (“descriptors” in columns) (de Bello et al. 2013). The functional dissimilarity between any two taxa varies between 0 (trait profiles of the two taxa are identical) and 1 (two taxa are completely dissimilar; i.e. used no common trait categories).
β-diversity
β-diversity represents the diversity that is found due to differences between communities in a given region. β-TD and β-FD were calculated as multivariate dispersions (Anderson et al. 2006). The calculated Rao matrices of TD- and FD-dissimilarities between each pair of samples at habitat scale (LW and control samples aggregated by substrate type and sampling campaign) (Eq. 1) were used to compute the distance of each sample to the centre of its group centroid (i.e. habitats) and to calculate the homogeneity of samples within LW and controls (betadisper function in “vegan” R package; Oksanen et al. 2018). We expect that the higher the dispersion among samples in each reach, the higher beta diversity will be in that group.
γ-diversity and diversity partitioning
γ-diversity indicates the diversity of a pooled set of samples, and it was obtained by pooling habitat samples of all reaches and river sections (Eq. 2; de Bello et al. 2010):
where S is the total number of species in the region and Pi the regional species relative abundance for species i. Pi is calculated as the average of pic (Eq. 1).
For diversity partitioning, β-diversity was calculated as the difference between γ-diversity and mean α (Eq. 3), and expressed as a percentage of the diversity of the whole study region (Eq. 4) (de Bello et al. 2010):
where, γ expresses the number of equivalent species at the regional scale, and mean α the average number of equivalent species at the sample scale (de Bello et al. 2010).
The Jost (2007) correction was applied to compute the Rao indices at α- and β-scales (de Bello et al. 2010).
Statistical analyses
The potential effects of habitat (LW vs. control samples merged across reaches and sampling campaigns), river substrates (gravel and sand sections), and their interaction, on α-TD and α-FD were investigated by two-way ANOVA (aov function from the “stats” R- package (R Core Team 2013)). The dispersion within habitat and river substrates on β-TD and β-FD was tested by two-way ANOVA (aov function from the “stats” R- package (R Core Team 2013)), using the multivariate distance to the centroid as the response variable.
Results
Habitat (LW and control) and river substrates (gravel and sand sections) had different effects on α-TD and α-FD across invertebrate assemblages (Table 2). Taxonomic diversity showed significant differences between habitats for hyporheic meiofauna (Table 2), with greater α-TD in LW than in control habitat, and in the gravel than in the sand river substrate (Fig. 3a). Taxonomic diversity of the macrofauna assemblages exhibited significant differences between river substrates (Table 2), with α-TD being greater in gravel for both benthic and hyporheic assemblages (Fig. 3b, c). α-FD had a similar response to α-TD for the hyporheic meiofauna by habitats and river substrates (Table 2, Fig. 3a). Hyporheic assemblages (macrofauna and meiofauna) showed different functional diversity between gravel and sandy river substrates, but only the functional diversity of the hyporheic meiofauna was significantly different between LW and control habitats (Fig. 3a, Table 2). In contrast, benthic macroinvertebrates were functionally similar between river substrates and habitats (Table 2). The interaction “habitat x river substrates” at α scale was not significant for any of the biodiversity metrics (Table 2).
For β-diversity, the effect of habitats and river substrates on taxonomic and functional dissimilarities of hyporheic meiofauna showed similar patterns to α- diversity (Table 2, Fig. 4a). β-TD and β-FD differed significantly by habitats and river substrates (Table 2), with greater β-TD and lower β-FD in LW than control, in both river substrates (Fig. 4a). Benthic macrofauna was characterized by significantly different β-TD in LW habitat, especially on sand river substrates (Table 2; Fig. 4c). Habitat types did not affect β-TD of hyporheic macrofauna (Table 2). β-FD was not significantly different between LW and control habitats for either benthic or hyporheic macrofaunal assemblages (Table 2).
γ-diversity showed large variation in taxonomic diversity across habitats and river substrates, while it was relatively constant for functional diversity (Fig. 5). γ-diversity for taxonomic diversity was generally lower in the sandy substrates; controls had lower diversity for hyporheic meiofauna and benthic macrofauna, but LW had lower diversity for hyporheic macrofauna (Fig. 5, 6). Interestingly, the relative contribution of α- and β-diversity to γ-diversity differed substantially between taxonomic and functional diversities (Fig. 5, 6). Most of the γ-diversity in TD was represented by β-diversity but it was α-diversity for FD (Fig. 6).
Discussion
This study assessed the effect of LW on taxonomic and functional diversities of invertebrate assemblages in a lowland river. It provides new empirical evidence on the structural or functional diversity of hyporheic and benthic assemblages at LW and across spatial scales.
At within-habitat scale (α-diversity), hyporheic meiofauna had significantly higher α-TD and α-FD in LW than in control habitat and was higher in gravel than in sand substrates. To our knowledge, only one other study has investigated hyporheic richness around LW (Wagenhoff and Olsen 2014). They observed higher density and lower diversity of hyporheic invertebrates in streams with LW and detected species-level preferences for wood. In our study, the meiofaunal assemblage in LW habitat was characterized by detritivores (Tanytarsini, Diamesinae), suggesting an increase of fine particulate food supplies and slow flow velocities around LW (Munn and Brusven 1991; Collier 1993) (Fig. 2a). This assemblage was also characterized by microcrustacean Cyclopoida that inhabit hard substrata covered by a thin layer of mud and detritus in slow flowing waters (Robertson et al. 1995; Dole-Olivier et al. 2000; Robertson 2000) (Fig. 2a). In support of our first hypothesis, i.e. taxonomic and functional diversities would be lower in control than LW habitat, α-FD and α-TD showed similar patterns indicating divergence in trait values within hyporheic meiofaunal assemblages, and suggesting greater within-sample variations in LW due to higher spatial heterogeneity (Vellend 2001). At within-habitat scale, LW may act as a filter for hyporheic trait values by increasing functional strategies of invertebrate assemblages (Magliozzi et al. 2019). Sand substrates supported lower α-diversity than gravel at both LW and control habitats (Fig. 3a). This result has been previously observed in other studies (Larsen and Ormerod 2010; Demars et al. 2012; White et al. 2017).
Relative contribution in percentage to mean abundance for a hyporheic meiofauna, b hyporheic macrofauna, and c benthic macrofauna in the Hammer stream on sampling occasions from October 2016 to August 2017. The ‘Others’ group (a) includes the taxa Amphipoda, Arhynchobdellida, Coleoptera, Diptera, Ephemeroptera, Plecoptera, Rhynchobdellida, Trichoptera, Tricladida, Trombidiformes, Truncatelloidea, Veneroida
Mean alpha diversity, represented as taxonomic (α-TD) and functional (α-FD) diversity, by habitats (control and large wood-LW) and river substrates (gravel and sand) for a hyporheic meiofauna, b hyporheic macrofauna, and c benthic macrofauna. Error bars are standard errors. Significant codes: ‘***’ ≤ 0.001, ‘**’ ≤ 0.01, ‘*’ ≤ 0.05, ‘ns’ > 0.05. Statistical significance between levels of river substrates and habitats is reported as ‘significance code river substrates/significance code habitat’. Note that y-axes differ between panels
For macrofaunal assemblages, α-TD and α-FD did not show significant differences between LW and control habitats for either hyporheic or benthic zones. This result contrasts with previous studies on benthic macroinvertebrates in lowland rivers that found a greater diversity in wood reaches (Smock et al. 1989; Benke and Wallace 2003; Pilotto et al. 2016). Higher α-TD in LW than in mineral habitats (i.e. bare gravel substrate) has been explained by the fact that organic biotopes like LW provide refuge from predators and a platform from which macroinvertebrates can consume detritus (Wharton et al. 2006). However, those previous studies used different analyses from ours to calculate α-TD. In our study, both hyporheic and benthic macrofaunal assemblages in LW habitat were characterized by taxa such as Gammarus pulex, Ephemera danica, Diamesinae, Tanytarsini and Oligochaeta, which are typical of habitats with high detritus content, and which likely feed on settled seston in low flow areas around LW (Collier 1993; Spänhoff and Meyer 2004; Pilotto et al. 2014; Cashman et al. 2016) (Table S1 in Online Resource 1, Fig. 2b, c). In the benthic zone, control habitat were also characterized by high abundances of Hydropsyche spp. and Limnius spp.. Some species of the Hydropsychidae (Trichoptera) are known to require stable substrates for attaching nets and maximizing food capture (Schröder et al. 2013; Pilotto et al. 2014). Regardless of river substrates, we observed similarity in functional traits of macroinvertebrate taxa between habitats (Fig. 3b, c), suggesting that LW supports taxa capable of performing similar functions (Larsen and Ormerod 2010). Such patterns may indicate that LW does not represent a limiting factor promoting trait divergence of macroinvertebrate assemblages in the Hammer Stream (Poff 1997).
At between-habitat scale (β-diversity), hyporheic meiofauna and benthic macrofauna assemblages showed significant functional and taxonomic dissimilarities between habitats (Table 2). Contrary to our second hypothesis, i.e. β-TD and β-FD differences would be higher at LW than control habitat, LW habitat showed greater taxonomic than functional dissimilarity (Fig. 4). The high dissimilarity in β-TD at LW may suggest that the presence of LW increases the heterogeneity of meiofaunal and benthic macrofaunal assemblages. In both gravel and sand, β-FD is far lower in LW than in control habitat, for the hyporheic meiofauna and macrofauna, and for the benthic macrofauna in the sandy substrate. A reduction in functional diversity can be linked to a reduction of specialist taxa exhibiting specialization for different trait categories, and to an increase in generalist taxa exhibiting affinity for various categories within traits. At this scale, the increased functional dissimilarity in control habitat indicates that LW might not behave as a driver of disturbance (Magliozzi et al. 2019), supporting divergence in trait values (Grime 2006), but as a filter causing trait convergence within invertebrate assemblages (Fig. 4a, c). This result broadly concurs with the fact that LW is not the only in-channel structure driving hyporheic exchange (e.g. in-channel vegetation, in-channel bedforms, riparian vegetation—Sawyer et al. 2012; Magliozzi et al. 2018). Therefore, in this study, the increase of β-TD at LW compartment (e.g. hyporheic and benthic) may be due to differences in small-scale environmental heterogeneity that provided local habitat benefits (Heino et al. 2012).
Results of betadisper function (“vegan” R package; Oksanen et al. 2018). Y axis reports the distance of each habitat to the centre of its group centroid (combination of LW and control, and gravel and sand) for a hyporheic meiofauna, b hyporheic macrofauna, and c benthic macrofauna. Error bars are standard error. Significant codes: ‘***’ ≤ 0.001, ‘**’ ≤ 0.01, ‘*’ ≤ 0.05, ‘ns’ > 0.05. Statistical significance between levels of river substrates and habitats is reported as ‘significance code river substrates/significance code habitat’. Note that y-axes differ between panels
γ-diversity revealed important differences between TD and FD (Fig. 5, 6). The proportion of γ-TD diversity was higher in LW than control habitat for meiofaunal and macrofaunal assemblages of the hyporheic and benthic zones (in the sand substrate). Therefore, our third hypothesis, i.e. the proportion of diversity explained by the variability between habitats would be higher in LW, was not supported for hyporheic macroinvertebrates. γ-FD and its spatial partitioning varied marginally between LW and control habitat suggesting high functional redundancy and independence from taxonomic diversity. Most of the functional variability was found to be at the within-habitat level, higher in LW than in control habitat, especially for hyporheic meiofauna, but this difference was very small for taxonomic diversity. This result is consistent with previous studies (White et al. 2017), and with the hypothesis that LW, acting as disturbance, is a strong driver of trait differentiation and species co-existence at the local habitat scale (MacArthur and Levins 1967; Grime 2006). In contrast to the diversifying effects of local LW disturbances, the taxonomic and functional diversities of invertebrates appear to be less variable on the gravel and sand dominant substrates. The convergence of traits may reflect the lowland environment of the Hammer Stream. The stream, as for many low-energy rivers, has limited geomorphic diversity, which might provide limited opportunities for the recruitment, establishment, growth and/or reproduction of a variety of species.
In conclusion, our study on a UK lowland river has shown that LW increases taxonomic and functional diversity mainly at the within-habitat scale and more widely for meiofauna. These results indicate a functional diversity that is decoupled from species diversity, showing a functional redundancy of macroinvertebrate assemblages in the Hammer Stream. The study also shows a functional variability at LW habitats, suggesting that species diversity is due to a great variability in environmental conditions proximal to LW. The consequences of trait diversification, as a result of LW disturbance, extend beyond minor adaptive shifts of phenological and behavioural traits linked with the capacity to exploit productive habitats (Magliozzi et al. 2019). Future research should move forward with improving the understanding of hydro-geomorphological impacts of LW on invertebrate assemblages, considering inter and intra annual variation of hyporheic and benthic invertebrates at wood sites.
References
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Beisel J-N, Usseglio-Polatera P, Thomas S, Moreteau J-C (1998) Stream community structure in relation to spatial variation: the influence of habitat characteristics. Hydrobiologia 389:73–88
Beisel J-N, Usseglio-Polatera P, Moreteau J-C (2000) The spatial heterogeneity of a river bottom: a key factor determining macroinvertebrate communities. Hydrobiologia 422(423):163–171
Benke AC, Wallace JB (2003) Influence of wood on invertebrate communities in streams and rivers. Am Fish Soc Symp 37:149–177
Bernot MJ, Dodds WK (2005) Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems 8:442–453
Botta-Dukát Z (2018) The generalized replication principle and the partitioning of functional diversity into independent alpha and beta components. Ecography 41:40–50
Boyer KL, Berg DR, Gregory SV (2003) Riparian management for wood in rivers. Am Fish Soc Symp 37:407–420
Cashman MJ, Pilotto F, Harvey GL, Wharton G, Pusch MT (2016) Combined stable-isotope and fatty-acid analyses demonstrate that large wood increases the autochthonous trophic base of a macroinvertebrate assemblage. Freshw Biol 61:549–564
Coleman MJ, Hynes HBN (1970) The vertical distribution of the invertebrate fauna in the bed of a stream. Limnol Oceanogr 15:31–40
Collier KJ (1993) Flow preferences of larval Chironomidae (Diptera) in Tongariro River, New Zealand. NZ J Mar Freshwat Res 27:219–226
Crossman J, Bradley C, Milner A, Pinay G (2013) Influence of environmental instability of groundwater-fed streams on hyporheic fauna, on a glacial floodplain, Denali National Park, Alaska. River Res Appl 29:548–559
de Bello F, Lepš J, Lavorel S, Moretti M (2007) Importance of species abundance for assessment of trait composition: an example based on pollinator communities. Community Ecol 8:163–170
de Bello F, Thuiller W, Lepš J, Choler P, Clement JC, Macek P, Sebastiá MT, Lavorel S (2009) Partitioning of functional diversity reveals the scale and extent of trait convergence and divergence. J Veg Sci 20:475–486
de Bello F, Lavergne S, Meynard CN, Lepš J, Thuiller W (2010) The partitioning of diversity: showing Theseus a way out of the labyrinth. J Veg Sci 21:992–1000
de Bello F, Carmona CP, Mason NW, Sebastià MT, Lepš J (2013) Which trait dissimilarity for functional diversity: trait means or trait overlap? J Veg Sci 24:807–819
De Castro-Català N, Muñoz I, Armendáriz L, Campos B, Barceló D, López-Doval J et al (2015) Invertebrate community responses to emerging water pollutants in Iberian river basins. Sci Total Environ 503:142–150
Demars BOL, Kemp JL, Friberg N, Usseglio-Polatera P, Harper DM (2012) Linking biotopes to invertebrates in rivers: Biological traits, taxonomic composition and diversity. Ecol Ind 23:301–311
Descloux S, Datry T, Usseglio-Polatera P (2014) Trait based structure of invertebrates along a gradient of sediment colmation: benthos versus hyporheos responses. Sci Total Environ 466(467):265–276
Diaz S, Cabido M, Casanoves F (1998) Plant functional traits and environmental filters at a regional scale. J Veg Sci 9:113–122
Díez JR, Larrañaga S, Elosegi A, Pozo J (2000) Effect of removal of wood on streambed stability and retention of organic matter. J N Am Benthol Soc 19:621–632
Dole-Olivier MJ, Galassi D, Marmonier P, Creuzé des Châtelliers M (2000) The biology and ecology of lotic microcrustaceans. Freshw Biol 44:63–91
Durkota JM, Wood PJ, Johns T, Thompson JR, Flower RJ (2019) Distribution of macroinvertebrate communities across surface and groundwater habitats in response to hydrological variability. Fund Appl Limnol/Arch Hydrobiol 193(1):79–92
Fanelli RM, Lautz LK (2008) Patterns of water, heat, and solute flux through streambeds around small dams. Groundwater 46:671–687
Fenchel T (1978) The ecology of micro and meiobenthos. Annu Rev Ecol Syst 9:9–121
Flores L, Larranaga A, Diez J, Elosegi A (2011) Experimental wood addition in streams: effects on organic matter storage and breakdown. Freshw Biol 56(10):2156–2167
Flores L, Díez JR, Larrañaga A, Pascoal C, Elosegi A (2013) Effects of retention site on breakdown of organic matter in a mountain stream. Freshw Biol 58(6):1267–1278
Gallardo B, Gascón S, Quintana X, Comín FA (2011) How to choose a biodiversity indicator–Redundancy and complementarity of biodiversity metrics in a freshwater ecosystem. Ecol Ind 11:1177–1184
Gippel CJ, O'neill IC, Finlayson BL, Schnatz IN (1996) Hydraulic guidelines for the re-introduction and management of large woody debris in lowland rivers. Regulated Rivers: Res Manage 12(2–3):223–236
Gower JC (1971) A general coefficient of similarity and some of its properties. Biometrics 27:857–871
Grabowski RC, Gurnell AM, Burgess-Gamble L, England J, Holland D, Klaar MJ et al (2019) The current state of the use of large wood in river restoration and management. Water Environ J. https://doi.org/10.1111/wej.12465
Gurnell A, Tockner K, Edwards P, Petts G (2005) Effects of deposited wood on biocomplexity of river corridors. Front Ecol Environ 3:377–382
Grime JP (2006) Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. J Veg Sci 17:255–260
Heino J, Grönroos M, Ilmonen J, Karhu T, Niva M, Paasivirta L (2012) Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate spatial scales. Freshw Sci 32:142–154
Hess KM, Wolf SH, Celia MA (1992) Large-scale natural gradient tracer test in sand and gravel, Cape Cod, Massachusetts: 3. hydraulic conductivity variability and calculated macrodispersivities. Water Resour Res 28:2011–2027
Hoffmann A, Hering D (2000) Wood-associated macroinvertebrate fauna in central European streams. Int Rev Hydrobiol 85:25–48
Jost L (2007) Partitioning diversity into independent alpha and beta components. Ecology 88:2427–2439
Kaller MD, Kelso WE (2007) Association of macroinvertebrate assemblages with dissolved oxygen concentration and wood surface area in selected subtropical streams of the southeastern USA. Aquat Ecol 41:95–110
Lepš J, de Bello F, Lavorel S, Berman S (2006) Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia 78:481–501
Larsen S, Ormerod SJ (2010) Combined effects of habitat modification on trait composition and species nestedness in river invertebrates. Biol Cons 143:2638–2646
Lautz LK, Siegel DI, Bauer RL (2006) Impact of debris dams on hyporheic interaction along a semi-arid stream. Hydrol Process 20:183–196
MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101(921):377–385
Magliozzi C, Grabowski RC, Packman AI, Krause S (2018) Toward a conceptual framework of hyporheic exchange across spatial scales. Hydrol Earth Syst Sci 22:6163–6185
Magliozzi C, Usseglio-Polatera P, Meyer A, Grabowski RC (2019) Functional traits of hyporheic and benthic invertebrates reveal importance of wood-driven geomorphological processes to rivers. Funct Ecol 33:1758–1770. https://doi.org/10.1111/1365-2435.13381
Marmonier P, Vervier P, Gibert J, Dole-Olivier MJ (1993) Biodiversity in ground waters. Trends Ecol Evol 8:392–395
Mouchet MA, Villéger S, Mason NW, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–876
Munn MD, Brusven MA (1991) Benthic macroinvertebrate communities in nonregulated and regulated waters of the Clearwater River, Idaho, USA. Regul Rivers Res Manag 6:1–11
Mutz M (2000) Influences of woody debris on flow patterns and channel morphology in a low energy, sand-bed stream reach. Int Rev Hydrobiol: J Covering Aspects Limnol Mar Biol 85(1):107–121
Mutz M, Rohde A (2003) Processes of surface-subsurface water exchange in a low energy sand-bed stream. Int Rev Hydrobiol 88:290–303
Mutz M, Kalbus E, Meinecke S (2007) Effect of instream wood on vertical water flux in low-energy sand bed flume experiments. Water Resour Res 43:W10424
Naegeli MW, Uehlinger U (1997) Contribution of the hyporheic zone to ecosystem metabolism in a prealpine gravel-bed-river. J N Am Benthol Soc 16:794–804
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al (2018) vegan: Community Ecology Package. R package version 2.4–6
Orghidan T (1959) Ein neuer Lebensraum des unterirdischen Wassers: der hyporheische Biotop. Arch Hydrobiol 55:392–414
Osei NA, Gurnell AM, Harvey GL (2015) The role of large wood in retaining fine sediment, organic matter and plant propagules in a small, single-thread forest river. Geomorphology 15(235):77–87
Pavoine S, Ollier S, Pontier D (2005) Measuring diversity from dissimilarities with Rao’s quadratic entropy: are any dissimilarities suitable? Theor Popul Biol 67:231–239
Petchey OL, Evans KL, Fishburn IS, Gaston KJ (2007) Low functional diversity and no redundancy in British avian assemblages. J Anim Ecol 76:977–985
Pilotto F, Bertoncin A, Harvey GL, Wharton G, Pusch MT (2014) Diversification of stream invertebrate communities by large wood. Freshw Biol 59:2571–2583
Pilotto F, Harvey GL, Wharton G, Pusch MT (2016) Simple large wood structures promote hydromorphological heterogeneity and benthic macroinvertebrate diversity in low-gradient rivers. Aquat Sci 78:755–766
Rao CR (1982) Diversity and dissimilarity coefficients—a unified approach. Theor Popul Biol 21:24–43
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Robertson AL (2000) Lotic meiofaunal community dynamics: colonisation, resilience and persistence in a spatially and temporally heterogeneous environment. Freshw Biol 44:135–147
Robertson AL, Wood P (2010) Ecology of the hyporheic zone: origins, current knowledge and future directions. Fund Appl Limnol/Arch Hydrobiol 176:279–289
Robertson AL, Lancaster J, Hildrew AG (1995) Stream hydraulics and the distribution of microcrustacea: a role for refugia? Freshw Biol 33:469–484
Sawyer AH, Cardenas BM, Buttles J (2012) Hyporheic temperature dynamics and heat exchange near channel-spanning logs. Water Resour Res 48:W01529
Schröder M, Kiesel J, Schattmann A, Jähnig SC, Lorenz AW, Kramm S et al (2013) Substratum associations of benthic invertebrates in lowland and mountain streams. Ecol Ind 30:178–189
Shelley F, Klaar M, Krause S, Trimmer M (2017) Enhanced hyporheic exchange flow around woody debris does not increase nitrate reduction in a sandy streambed. Biogeochemistry 136:353–372
Smock LA, Metzler GM, Gladden JE (1989) Role of debris dams in the structure and functioning of low-gradient headwater streams. Ecology 70:764–775
Smock LA, Gladden JE, Riekenberg JL, Smith LC, Black CR (1992) Lotic macroinvertebrate production in three dimensions: channel surface, hyporheic, and floodplain environments. Ecology 73:876–886
Spänhoff B, Meyer EI (2004) Breakdown rates of wood in streams. J N Am Benthol Soc 23:189–197
Stofleth JM, Shields FD Jr, Fox GA (2008) Hyporheic and total transient storage in small, sand-bed streams. Hydrol Processes: Int J 22(12):1885–1894
Sutfin NA, Wohl EE, Dwire KA (2016) Banking carbon: a review of organic carbon storage and physical factors influencing retention in floodplains and riparian ecosystems. Earth Surf Proc Land 41(1):38–60
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertébrés d’eau douce, 2nd edn. CNRS éditions, Paris
Thevenet A, Citterio A, Piégay H (1998) A new methodology for the assessment of large woody debris accumulations on highly modified rivers (example of two French Piedmont rivers). Regul Rivers Res Manag 14:467–483
Thompson MS, Brooks SJ, Sayer CD, Woodward G, Axmacher JC, Perkins DM, Gray C (2018) Large woody debris “rewilding” rapidly restores biodiversity in riverine food webs. J Appl Ecol 55:895–904
Vellend M (2001) Do commonly used indices of beta-diversity measure species turnover? J Veg Sci 12:545–552
Wagenhoff A, Olsen D (2014) Does large woody debris affect the hyporheic ecology of a small New Zealand pasture stream? NZ J Mar Freshwat Res 48:547–559
Wharton G, Cotton JA, Wotton RS, Bass JAB, Heppell CM, Trimmer M, Sanders IA, Warren LL (2006) Macrophytes and suspension- feeding invertebrates modify flows and fine sediments in the Frome and Piddle catchments, Dorset (UK). J Hydrol 330:171–184
White JC, Hill MJ, Bickerton MA, Wood PJ (2017) Macroinvertebrate taxonomic and functional trait compositions within lotic habitats affected by river restoration practices. Environ Manag 60:513–525
Wohl E (2013) Floodplains and wood. Earth Sci Rev 123:194–212
Wohl E, Cenderelli DA, Dwire KA, Ryan-Burkett SE, Young MK, Fausch KD (2010) Large in-stream wood studies: a call for common metrics. Earth Surf Proc Land 35:618–625
Acknowledgements
This research was funded under the European Union’s Marie Sklodowska-Curie Action, Horizon2020 within the HypoTRAIN project (Grant Agreement Number 641939). We thank Dr. Carlos P. Carmona for the helpful discussion on the TD and FD approaches.
Author information
Authors and Affiliations
Contributions
CM designed, collected the data, analysed the dataset and wrote the manuscript. Co-authors provided guidance on statistical analyses, structure, and editing on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magliozzi, C., Meyer, A., Usseglio-Polatera, P. et al. Investigating invertebrate biodiversity around large wood: taxonomic vs functional metrics. Aquat Sci 82, 69 (2020). https://doi.org/10.1007/s00027-020-00745-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-00745-9