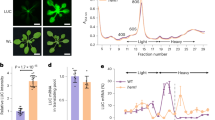
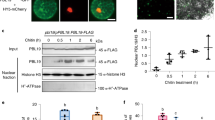
Abstract
The survival of all living organisms requires the ability to detect attacks and swiftly counter them with protective immune responses. Despite considerable mechanistic advances, the interconnectivity of signalling modules often remains unclear. A newly characterized protein, IMMUNOREGULATORY RNA-BINDING PROTEIN (IRR), negatively regulates immune responses in both maize and Arabidopsis, with disrupted function resulting in enhanced disease resistance. IRR associates with and promotes canonical splicing of transcripts encoding defence signalling proteins, including the key negative regulator of pattern-recognition receptor signalling complexes, CALCIUM-DEPENDENT PROTEIN KINASE 28 (CPK28). On immune activation by Plant Elicitor Peptides (Peps), IRR is dephosphorylated, disrupting interaction with CPK28 transcripts and resulting in the accumulation of an alternative splice variant encoding a truncated CPK28 protein with impaired kinase activity and diminished function as a negative regulator. We demonstrate a new mechanism linking Pep-induced post-translational modification of IRR with post-transcriptionally mediated attenuation of CPK28 function to dynamically amplify Pep signalling and immune output.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw read sequences are deposited in the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE146282. The data generated and analysed in this study are included in the published article and Supplementary Information. All data are available from the corresponding author upon request.
References
Kobayashi, K. S. & Flavell, R. A. Shielding the double-edged sword: negative regulation of the innate immune system. J. Leukoc. Biol. 75, 428–433 (2004).
Gassmann, W. Alternative splicing in plant defense. Curr. Top. Microbiol. Immunol. 326, 219–233 (2008).
Liu, J., Qian, C. & Cao, X. Post-translational modification control of innate immunity. Immunity 45, 15–30 (2016).
Xu, G. et al. Global translational reprogramming is a fundamental layer of immune regulation in plants. Nature 545, 487–490 (2017).
Nuhse, T. S., Bottrill, A. R., Jones, A. M. & Peck, S. C. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 51, 931–940 (2007).
Withers, J. & Dong, X. Post-translational regulation of plant immunity. Curr. Opin. Plant Biol. 38, 124–132 (2017).
Tena, G., Boudsocq, M. & Sheen, J. Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 14, 519–529 (2011).
Lu, D. et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439–1442 (2011).
Feng, B. et al. Protein poly(ADP-ribosyl)ation regulates Arabidopsis immune gene expression and defense responses. PLoS Genet. 11, e1004936 (2015).
Macho, A. P. & Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 54, 263–272 (2014).
Yu, X., Feng, B., He, P. & Shan, L. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 55, 109–137 (2017).
Huffaker, A., Pearce, G. & Ryan, C. A. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl Acad. Sci. USA 103, 10098–10103 (2006).
Huffaker, A., Dafoe, N. J. & Schmelz, E. A. ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 155, 1325–1338 (2011).
Huffaker, A. et al. Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc. Natl Acad. Sci. USA 110, 5707–5712 (2013).
Trivilin, A. P., Hartke, S. & Moraes, M. G. Components of different signalling pathways regulated by a new orthologue of AtPROPEP1 in tomato following infection by pathogens. Plant Pathol. 63, 1110–1118 (2014).
Lee, M. W., Huffaker, A., Crippen, D., Robbins, R. T. & Goggin, F. L. Plant elicitor peptides promote plant defences against nematodes in soybean. Mol. Plant Pathol. 19, 858–869 (2018).
Ruiz, C., Nadal, A., Montesinos, E. & Pla, M. Novel Rosaceae plant elicitor peptides as sustainable tools to control Xanthomonas arboricola pv. pruni in Prunus spp. Mol. Plant Pathol. 19, 418–431 (2018).
Lori, M. et al. Evolutionary divergence of the plant elicitor peptides (Peps) and their receptors: interfamily incompatibility of perception but compatibility of downstream signalling. J. Exp. Bot. 66, 5315–5325 (2015).
Hander, T. et al. Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides. Science 363, eaar7486 (2019).
Yamaguchi, Y., Pearce, G. & Ryan, C. A. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl Acad. Sci. USA 103, 10104–10109 (2006).
Yamaguchi, Y., Huffaker, A., Bryan, A. C., Tax, F. E. & Ryan, C. A. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22, 508–522 (2010).
Krol, E. et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 285, 13471–13479 (2010).
Tintor, N. et al. Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc. Natl Acad. Sci. USA 110, 6211–6216 (2013).
Ross, A. et al. The Arabidopsis PEPR pathway couples local and systemic plant immunity. EMBO J. 33, 62–75 (2014).
Postel, S. et al. The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur. J. Cell Biol. 89, 169–174 (2010).
Liu, Z. et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc. Natl Acad. Sci. USA 110, 6205–6210 (2013).
Lu, D. et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl Acad. Sci. USA 107, 496–501 (2010).
Schulze, B. et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285, 9444–9451 (2010).
Roux, M. et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23, 2440–2455 (2011).
Li, L. et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338 (2014).
Lal, N. K. et al. The receptor-like cytoplasmic kinase BIK1 localizes to the nucleus and regulates defense hormone expression during plant innate immunity. Cell Host Microbe 23, 485–497 (2018).
Monaghan, J. et al. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16, 605–615 (2014).
Wang, J. et al. A regulatory module controlling homeostasis of a plant immune kinase. Mol. Cell 69, 493–504 (2018).
Walley, J. W. et al. Integration of omic networks in a developmental atlas of maize. Science 353, 814–818 (2016).
Golovkin, M. & Reddy, A. S. An SC35-like protein and a novel serine/arginine-rich protein interact with Arabidopsis U1-70K protein. J. Biol. Chem. 274, 36428–36438 (1999).
Carvalho, R. F. et al. The Arabidopsis SR45 splicing factor, a negative regulator of sugar signaling, modulates SNF1-related protein kinase 1 stability. Plant Cell 28, 1910–1925 (2016).
Zhang, X. N. et al. Transcriptome analyses reveal SR45 to be a neutral splicing regulator and a suppressor of innate immunity in Arabidopsis thaliana. BMC Genomics 18, 772 (2017).
Kadota, Y. et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54, 43–55 (2014).
Asai, T. et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 (2002).
Zhang, M., Su, J., Zhang, Y., Xu, J. & Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 45, 1–10 (2018).
Mei, Y., Zhang, C., Kernodle, B. M., Hill, J. H. & Whitham, S. A. A Foxtail mosaic virus vector for virus-induced gene silencing in maize. Plant Physiol. 171, 760–772 (2016).
Anko, M. L. Regulation of gene expression programmes by serine–arginine rich splicing factors. Semin. Cell Dev. Biol. 32, 11–21 (2014).
Jeong, S. SR proteins: binders, regulators, and connectors of RNA. Mol. Cell 40, 1–9 (2017).
Thines, B. et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665 (2007).
Chung, H. S. & Howe, G. A. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 21, 131–145 (2009).
Jabs, T., Tschöpe, M., Colling, C., Hahlbrock, K. & Scheel, D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl Acad. Sci. USA 94, 4800–4805 (1997).
Acharya, B. R. et al. Overexpression of CRK13, an Arabidopsis cysteine-rich receptor-like kinase, results in enhanced resistance to Pseudomonas syringae. Plant J. 50, 488–499 (2007).
Waese, J. et al. ePlant: visualizing and exploring multiple levels of data for hypothesis generation in plant biology. Plant Cell 29, 1806–1821 (2017).
Ali, G. S. et al. Regulation of plant developmental processes by a novel splicing factor. PLoS ONE 2, e471 (2007).
Liese, A. & Romeis, T. Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophys. Acta 1833, 1582–1589 (2013).
Klimecka, M. & Muszynska, G. Structure and functions of plant calcium-dependent protein kinases. Acta Biochim. Pol. 54, 219–233 (2007).
Manley, J. L. & Tacke, R. SR proteins and splicing control. Genes Dev. 10, 1569–1579 (1996).
Xing, D., Wang, Y., Hamilton, M., Ben-Hur, A. & Reddy, A. S. Transcriptome-wide identification of RNA targets of Arabidopsis SERINE/ARGININE-RICH45 uncovers the unexpected roles of this RNA binding protein in RNA processing. Plant Cell 27, 3294–3308 (2015).
Carpenter, S., Ricci, E. P., Mercier, B. C., Moore, M. J. & Fitzgerald, K. A. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol. 14, 361–376 (2014).
Yang, S., Tang, F. & Zhu, H. Alternative splicing in plant immunity. Int. J. Mol. Sci. 15, 10424–10445 (2014).
Howard, B. E. et al. High-throughput RNA sequencing of Pseudomonas-infected Arabidopsis reveals hidden transcriptome complexity and novel splice variants. PLoS ONE 8, e74183 (2013).
Ling, Z., Zhou, W., Baldwin, I. T. & Xu, S. Insect herbivory elicits genome-wide alternative splicing responses in Nicotiana attenuata. Plant J. 84, 228–243 (2015).
Bazin, J. et al. Role of MPK4 in pathogen-associated molecular pattern-triggered alternative splicing in Arabidopsis. PLoS Pathog. 16, e1008401 (2020).
Xu, S. et al. Transportin-SR is required for proper splicing of resistance genes and plant immunity. PLoS Genet. 7, e1002159 (2011).
Zhang, Z. et al. Splicing of receptor-like kinase-encoding SNC4 and CERK1 is regulated by two conserved splicing factors that are required for plant immunity. Mol. Plant 7, 1766–1775 (2014).
Huang, J. et al. An oomycete plant pathogen reprograms host pre-mRNA splicing to subvert immunity. Nat. Commun. 8, 2051 (2017).
Dinesh-Kumar, S. P. & Baker, B. J. Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc. Natl Acad. Sci. USA 97, 1908–1913 (2000).
Zhang, X. C. & Gassmann, W. Alternative splicing and mRNA levels of the disease resistance gene RPS4 are induced during defense responses. Plant Physiol. 145, 1577–1587 (2007).
Liu, J. et al. Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol. 171, 1427–1442 (2016).
Feng, F. et al. A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485, 114–118 (2012).
Rao, S. et al. Roles of receptor-like cytoplasmic kinase VII members in pattern-triggered immune signaling. Plant Physiol. 177, 1679–1690 (2018).
Walley, J. W. et al. Reconstruction of protein networks from an atlas of maize seed proteotypes. Proc. Natl Acad. Sci. USA 110, E4808–E4817 (2013).
Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. REVIGO summarizes and visualizes long lists of Gene Ontology terms. PLoS ONE 6, e21800 (2011).
Gietz, R. D. & Woods, R. A. in Yeast Protocol (ed. Xiao, W.) 107–120 (Humana, 2006).
Schwessinger, B. et al. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 7, e1002046 (2011).
Xu, F. & Copeland, C. Nuclear extraction from Arabidopsis thaliana. Bio. Protoc. 2, e306 (2012).
Schmelz, E. A., Alborn, H. T. & Tumlinson, J. H. The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214, 171–179 (2001).
Acknowledgements
We thank S. A. Whitham (Iowa State University Plant Sciences Institute) for providing the constructs for the VIGS experiments, and A. Groisman (University of California San Diego Department of Physics) for the use of his Biolistic inoculation apparatus. This work was funded by NSF CAREER Award no. 1943591, a Hellman Foundation Fellowship and UC San Diego Start-up funds to A.H. K.D. was additionally funded by Ciências sem Fronteiras/CNPq fellowship no. 200260/2015‐4. E.P. was additionally funded by the Cell and Molecular Genetics (CMG) Training Program at the University of California, San Diego. Z.S. and S.P.B. were funded by NSF award no. 1546899.
Author information
Authors and Affiliations
Contributions
A.H. and K.D. conceived the project. K.D. conducted the experiments. A.H. and K.D. analysed the data and wrote the manuscript. P.R.W. performed the MAP kinase assay, confocal microscopy experiments and phylogenetic analysis. E.P. analysed the leaf volatile emissions and helped edit the figures. Y.T. performed the in-gel kinase activity assays. C.V. assisted with the generation of the transgenic plant lines. Z.S. and S.P.B. performed the phosphoproteomic analysis. J.I.S. contributed critical experimental resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, references and methods.
Supplementary Tables
Supplementary Table 1: differentially expressed genes (DEG); Supplementary Table 2: upregulated genes in irr-1 compared with the wild type; Supplementary Table 3: alternative splicing analysis; Supplementary Table 4: list of primers used.
Rights and permissions
About this article
Cite this article
Dressano, K., Weckwerth, P.R., Poretsky, E. et al. Dynamic regulation of Pep-induced immunity through post-translational control of defence transcript splicing. Nat. Plants 6, 1008–1019 (2020). https://doi.org/10.1038/s41477-020-0724-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0724-1
This article is cited by
-
Reactive oxygen species in plants: an invincible fulcrum for biotic stress mitigation
Applied Microbiology and Biotechnology (2022)
-
Sp(l)icing up PepR signalling
Nature Plants (2020)