Abstract
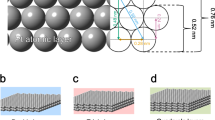
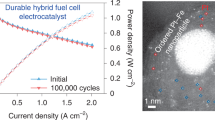
A remaining challenge for the deployment of proton-exchange membrane fuel cells is the limited durability of platinum (Pt) nanoscale materials that operate at high voltages during the cathodic oxygen reduction reaction. In this work, atomic-scale insight into well-defined single-crystalline, thin-film and nanoscale surfaces exposed Pt dissolution trends that governed the design and synthesis of durable materials. A newly defined metric, intrinsic dissolution, is essential to understanding the correlation between the measured Pt loss, surface structure, size and ratio of Pt nanoparticles in a carbon (C) support. It was found that the utilization of a gold (Au) underlayer promotes ordering of Pt surface atoms towards a (111) structure, whereas Au on the surface selectively protects low-coordinated Pt sites. This mitigation strategy was applied towards 3 nm Pt3Au/C nanoparticles and resulted in the elimination of Pt dissolution in the liquid electrolyte, which included a 30-fold durability improvement versus 3 nm Pt/C over an extended potential range up to 1.2 V.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data are available in the main text and in the Supplementary Information, and are also available from corresponding author upon reasonable request. Source data for the bar graphs are provided with this paper.
Change history
15 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41563-020-0782-9
References
Turner, J. A. Sustainable hydrogen production. Science 305, 972–974 (2004).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2016).
Eberle, U. & Von Helmolt, R. Sustainable transportation based on electric vehicle concepts: a brief overview. Energy Environ. Sci. 3, 689–699 (2010).
Yoshida, T. & Kojima, K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Electrochem. Soc. Interface 24, 45–49 (2015).
Gittleman, C. S., Kongkanand, A., Masten, D. & Gu, W. Materials research and development focus areas for low cost automotive proton-exchange membrane fuel cells. Curr. Opin. Electrochem 18, 81–89 (2019).
Bu, L. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Stephens, I. E. L., Rossmeisl, J. & Chorkendorff, I. Toward sustainable fuel cells. Science 354, 1378–1379 (2016).
Wang, L. et al. Tunable intrinsic strain in two-dimensional transition metal electrocatalysts. Science 363, 870–874 (2019).
Chattot, R. et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 17, 827–833 (2018).
Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016).
Kang, Y. et al. Multimetallic core/interlayer/shell nanostructures as advanced electrocatalysts. Nano Lett. 14, 6361–6367 (2014).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2, 454–460 (2010).
Ye, X., Ruban, A. V. & Mavrikakis, Manos Adsorption and dissociation of O2 on Pt–Co and Pt–Fe alloys. J. Am. Chem. Soc. 126, 4717–4725 (2004).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Ferreira, P. J. et al. Instability of Pt/C electrocatalysts in proton exchange membrane fuel cells: a mechanistic investigation. J. Electrochem. Soc. 152, 2256–2271 (2005).
Shao-Horn, Y. et al. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 46, 285–305 (2007).
Borup, R. et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 107, 3904–3951 (2007).
Mayrhofer, K. J. J. et al. Non-destructive transmission electron microscopy study of catalyst degradation under electrochemical treatment. J. Power Sources 185, 734–739 (2008).
Mayrhofer, K. J. J. et al. Fuel cell catalyst degradation on the nanoscale. Electrochem. Commun. 10, 1144–1147 (2008).
Takahashi, I. & Kocha, S. S. Examination of the activity and durability of PEMFC catalysts in liquid electrolytes. J. Power Sources 195, 6312–6322 (2010).
Wang, X., Kumar, R. & Myers, D. J. Effect of voltage on platinum dissolution relevance to polymer electrolyte fuel cells. Electrochem. Solid-State Lett. 9, 225–227 (2006).
Pizzutilo, E. et al. On the need of improved accelerated degradation protocols (ADPs): examination of platinum dissolution and carbon corrosion in half-cell tests. J. Electrochem. Soc. 163, F1510–F1514 (2016).
Meier, J. C. et al. Degradation mechanisms of Pt/C fuel cell catalysts under simulated start–stop conditions. ACS Catal. 2, 832–843 (2012).
Hodnik, N. et al. Severe accelerated degradation of PEMFC platinum catalyst: a thin film IL-SEM study. Electrochem. Commun. 30, 75–78 (2013).
Uchimura, M. & Kocha, S. The impact of cycle profile on PEMFC durability. ECS Trans. 11, 1215–1226 (2007).
Wilson, M. S., Garzon, F. H., Gottesfeld, S. & Kurt, E. Surface area loss of supported platinum in polymer electrolyte fuel cells. J. Electrochem. Soc. 140, 2872–2877 (1993).
Smith, M. C., Gilbert, J. A., Mawdsley, J. R., Seifert, S. & Myers, D. J. In situ small-angle X-ray scattering observation of Pt catalyst particle growth during potential cycling. J. Am. Chem. Soc. 130, 8112–8113 (2008).
Li, D. et al. Functional links between Pt single crystal morphology and nanoparticles with different size and shape: the oxygen reduction reaction case. Energy Environ. Sci. 7, 4061–4069 (2014).
Tang, L. et al. Electrochemical stability of nanometer-scale Pt particles in acidic environments. J. Am. Chem. Soc. 132, 596–600 (2010).
Tang, L., Li, X., Cammarata, R. C., Friesen, C. & Sieradzki, K. Electrochemical stability of elemental metal nanoparticles. J. Am. Chem. Soc. 132, 11722–11726 (2010).
Topalov, A. A. et al. Dissolution of platinum: limits for the deployment of electrochemical energy conversion? Angew. Chem. Int. Ed. 51, 12613–12615 (2012).
Lopes, P. P. et al. Relationships between atomic level surface structure and stability/activity of platinum surface atoms in aqueous environments. ACS Catal. 6, 2536–2544 (2016).
Cherevko, S. et al. Dissolution of platinum in the operational range of fuel cells. ChemElectroChem 2, 1471–1478 (2015).
Lopes, P. P. et al. Dynamics of electrochemical Pt dissolution at atomic and molecular levels. J. Electroanal. Chem. 819, 123–129 (2018).
Pavlišič, A. et al. Platinum dissolution and redeposition from Pt/C fuel cell electrocatalyst at potential cycling. J. Electrochem. Soc. 165, F3161–F3165 (2018).
Jacobse, L., Huang, Y. F., Koper, M. T. M. & Rost, M. J. Correlation of surface site formation to nanoisland growth in the electrochemical roughening of Pt(111). Nat. Mater. 17, 277–282 (2018).
Ott, S. et al. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 19, 77–85 (2020).
Sui, S. et al. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 5, 1808–1825 (2017).
Markovic, N. M. & Ross, P. N. Jr. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 45, 117–229 (2002).
Van Der Vliet, D. F. et al. Mesostructured thin films as electrocatalysts with tunable composition and surface morphology. Nat. Mater. 11, 1051–1058 (2012).
Snyder, J. et al. Thin film approach to single crystalline electrochemistry. J. Phys. Chem. C 117, 23790–23796 (2013).
Kinoshita, K. Electrochemical Oxygen Technology (John Wiley & Sons, 1992).
Bi, W., Gray, G. E. & Fuller, T. F. PEM fuel cell PtC dissolution and deposition in Nafion electrolyte. Electrochem. Solid-State Lett. 10, 101–104 (2007).
Macauley, N. et al. Pt band formation enhances the stability of fuel cell membranes. ECS Electrochem. Lett. 2, 33–35 (2013).
Zhang, J., Sasaki, K., Sutter, E. & Adzic, R. R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 315, 220–222 (2007).
Kodama, K., Jinnouchi, R., Takahashi, N., Murata, H. & Morimoto, Y. Activities and stabilities of Au-modified stepped-Pt single-crystal electrodes as model cathode catalysts in polymer electrolyte fuel cells. J. Am. Chem. Soc. 138, 4194–4200 (2016).
Wang, C. et al. Multimetallic Au/FePt3 nanoparticles as highly durable electrocatalyst. Nano Lett. 11, 919–926 (2011).
Ruban, A. V., Skriver, H. L. & Nørskov, J. K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 59, 15990–16000 (1999).
Strmcnik, D. et al. When small is big: the role of impurities in electrocatalysis. Top. Catal. 58, 1174–1180 (2015).
Li, D. et al. Surfactant removal for colloidal nanoparticles from solution synthesis: the effect on catalytic performance. ACS Catal. 2, 1358–1362 (2012).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Haynes, W. M., Bruno, T. J., Lide, D. R. in CRC Handbook of Chemistry and Physics 95th edn (ed. Haynes, W. M.) 145–152 (CRC, 2015).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Acknowledgements
This work was done at the Argonne National Laboratory, which is operated for the US Department of Energy (DOE) Office of Science by the UCArgonne, LLC, under contract no. DE-AC02-06CH11357. The research efforts on single-crystalline systems, well-defined thin films and in situ dissolution measurements were supported by the Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division. Synthesis and characterization of the nanoscale materials was supported by the US DOE Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office. Transmission electron microscopy studies were accomplished at the Center for Nanoscale Materials at the Argonne National Laboratory, an Office of Science user facility supported by the US DOE Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02- 06CH11357, and at the Center for Nanophase Materials Sciences at Oak Ridge National Laboratory, an Office of Science user facility supported by the US DOE Office of Science, Office of Basic Energy Sciences, with work supported by the Hydrogen & Fuel Cell Technologies Office, Energy Efficiency and Renewable Energy, US Department of Energy. Computational modelling work at University of Wisconsin-Madison was supported by the Department of Energy, Basic Energy Sciences, Division of Chemical Sciences (grant DE-FG02-05ER15731), and was partially performed using supercomputer resources at the National Energy Research Scientific Computing Center (NERSC). NERSC is supported by the US DOE, Office of Science, under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
P.P.L., H.D., C.W. and V.R.S. conceived the idea and designed the experiments. P.P.L. performed in situ dissolution measurements on all the systems. C.W., D.L. and J.S. developed and performed thin-film depositions. H.L., Y.K., N.B. and D.L. performed the synthesis and characterization of nanoscale materials. M.M. and R.S. designed and performed the theoretical modelling work. D.T. and Y.S. performed STM and AFM measurements. K.L.M. performed the HR-STEM and EDS characterizations. P.P.L., D.L., D.S., M.M., N.M.M. and V.R.S. analysed and discussed the results. C.W., P.P.L. and V.R.S. drafted the manuscript. V.R.S. supervised the research. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Figs. 1–13, captions and notes for Tables 1 and 2 and Figs. 1–13 and references 1–6.
Source data
Source Data Fig. 1
Numerical data used to generate bar graphs displayed in panels g, h, and i.
Source Data Fig. 2
Numerical data used to generate bar graph displayed in panel f.
Source Data Fig. 3
Numerical data used to generate graph displayed in panel c.
Source Data Fig. 4
Numerical data used to generate bar graph data displayed in panel h.
Rights and permissions
About this article
Cite this article
Lopes, P.P., Li, D., Lv, H. et al. Eliminating dissolution of platinum-based electrocatalysts at the atomic scale. Nat. Mater. 19, 1207–1214 (2020). https://doi.org/10.1038/s41563-020-0735-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0735-3
This article is cited by
-
Emerging Atomically Precise Metal Nanoclusters and Ultrasmall Nanoparticles for Efficient Electrochemical Energy Catalysis: Synthesis Strategies and Surface/Interface Engineering
Electrochemical Energy Reviews (2024)
-
Mesoporous Pt@Pt-skin Pt3Ni core-shell framework nanowire electrocatalyst for efficient oxygen reduction
Nature Communications (2023)
-
Probing the atomically diffuse interfaces in Pd@Pt core-shell nanoparticles in three dimensions
Nature Communications (2023)
-
Mechanistic insights on Bi-potentiodynamic control towards atomistic synthesis of electrocatalysts for hydrogen evolution reaction
Scientific Reports (2023)
-
PtCu subnanoclusters epitaxial on octahedral PtCu/Pt skin matrix as ultrahigh stable cathode electrocatalysts for room-temperature hydrogen fuel cells
Nano Research (2023)