Abstract
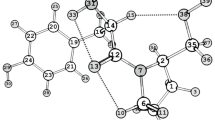
The kinetics of the reaction of L-tyrosyl-L-proline (Tyr–Pro) with di- and trinitrophenyl benzoates in aqueous 1,4-dioxane (40 wt % of water) were studied in the temperature range 298–313 K. The reaction rate constant k298 was found to vary in the range from 0.035 to 0.564 L·mol–1·s–1, and the activation barrier changed from 39 to 51 kJ/mol. The neutral and anionic forms of the dipeptide were simulated by the DFT/B3LYP/ cc-pVTZ method. Natural bond orbital analysis of the electron density distribution in these forms showed that the preferred nucleophilic center in the dipeptide molecule is the nitrogen atom of the primary amino group rather than the oxygen atom of the phenolic hydroxy group.





Similar content being viewed by others
REFERENCES
Antipin, I.S., Kazymova, M.A., Kuznetsov, M.A., Vasilyev, A.V., Ishchenko, M.A., Kiryushkin, A.A., Kuznetsova, L.M., Makarenko, S.V., Ostrovskii, V.A., Petrov, M.L., Solod, O.V., Trishin, Yu.G., Yakovlev, I.P., Nenaidenko, V.G., Beloglazkina, E.K., Beletskaya, I.P., Ustynyuk, Yu.A., Solov’ev, P.A., Ivanov, I.V., Malina, E.V., Sivova, N.V., Negrebetskii, V.V., Baukov, Yu.I., Pozharskaya, N.A., Traven’, V.F., Shchekotikhin, A.E., Varlamov, A.V., Borisova, T.N., Lesina, Yu.A., Krasnokutskaya, E.A., Rogozhnikov, S.I., Shurov, S.N., Kustova, T.P., Klyuev, M.V., Khelevina, O.G., Stuzhin, P.A., Fedorov, A.Yu., Gushchin, A.V., Dodonov, V.A., Kolobov, A.V., Plakhtinskii, V.V., Orlov, V.Yu., Kriven’ko, A.P., Fedotova, O.V., Pchelintseva, N.V., Charushin, V.N., Chupakhin, O.N., Klimochkin, Yu.N., Klimochkina, A.Yu., Kuryatnikov, V.N., Malinovskaya, Yu.A., Levina, A.S., Zhuravlev, O.E., Voronchikhina, L.I., Fisyuk, A.S., Aksenov, A.V., Aksenov, N.A., and Aksenova, I.V., Russ. J. Org. Chem., 2017, vol. 53, p. 1275. https://doi.org/10.1134/S1070428017090019
Kuritsyn, L.V., Kustova, T.P., Sadovnikov, A.I., Kalinina, N.V., and Klyuev, M.V., Kinetika reaktsii atsil’nogo perenosa (Kinetics of Acyl Group Transfer Reactions), Kuritsyn, L.V., Ed., Ivanovo: Ivanov. Gos. Univ., 2006.
Kuritsyn, L.V., Kochetova, L.B., Kalinina, N.V., and Kustova, T.P.,Russ. J. Gen. Chem., 2012, vol. 82, p. 1805. https://doi.org/10.1134/S1070363212110114
Kochetova, L.B., Kalinina, N.V., Grabchileva, Yu.E., Simonova, K.A., and Kustova, T.P., Butlerov. Soobshch., 2015, vol. 43, p. 1.
Guzevatykh, L.S., Voronina, T.A., Emel’yanova, T.G., Seredenin, S.B., Andreeva, L.A., Alfeeva, L.Yu., and Myasoedov, N.F., Biology Bull., 2008, vol. 35, p. 50–55. https://doi.org/10.1134/S1062359008010081
Guzevatykh, L.S., Russ. J. Bioorg. Chem., 2008, vol. 34, p. 526. https://doi.org/10.1134/S1068162008050026
The IUPAC Stability Constants Database (SC-Database)©, software version 5.86, data version 4.83.
Frisch M.J., Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision B.04, Wallingford CT: Gaussian, 2003.
Glendening, E.D., Reed, A.E., Carpenter, J.E., and Weinhold, F.,QCPE Bull., 1990, vol. 10, p. 58.
Weinhold, F. and Landis, C.R., Valency and Bonding. A Natural Bond Orbital Donor–Acceptor Perspective. Cambridge UK: Cambridge Univ. Press, 2005.
ACKNOWLEDGMENTS
The authors thank Prof. Giricheva N.I. for her help in performing quantum chemical calculations and discussing their results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kustova, T.P., Lokteva, I.I., Kochetova, L.B. et al. Reactivity of Tyrosyl–Proline toward Benzoylation in Aqueous 1,4-Dioxane. Russ J Org Chem 56, 1034–1040 (2020). https://doi.org/10.1134/S1070428020060111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020060111