Abstract
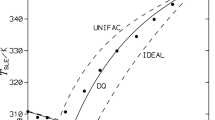
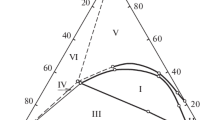
The phase diagrams of the liquid systems RnSi(OR’)4−n-Me2CO-H2O and RnSiCl4−n-Me2CO-H2O, where R = Me, Ph; R’ = Me, Et; n = 0–2, were compared. Mathematical modeling of the phase equilibrium in these systems was performed on the basis of the UNIFAC and NRTL equations. The heterogeneous regions, conodes of phase equilibrium, and compositions of the coexisting phases are determined. A satisfactory description of the experimental data by the parameters of the models of phase equilibrium obtained on the basis of the NRTL equations is shown for the phase diagrams of the MeSi(OEt)3/Si(OEt)4-Me2CO-H2O systems. The heterophase character due to the mutual insolubility of the monomers and water is common for both systems. The heterogeneous region of the RnSi(OR’)4−n-Me2CO-H2O system is smaller than that of RnSiCl4−n-Me2CO-H2O (calculation by the UNIFAC method). At the same acetone concentration, the water concentration in the organic phases of the “alkoxy” systems is higher than that in the “chlorosilane” systems. The replacement of acetone with ethanol and AcOH leads to a sharp reduction of the heterogeneous region.
Similar content being viewed by others
References
L. B. Sokolov, Polikondensatsionnyi metod sinteza polimerov [Polycondensation Method for Polymer Synthesis], Khimiya, Moscow, 1966, 332 pp. (in Russian).
P. V. Ivanov, D. N. Golubykh, E. A. Chernyshev, Russ. Chem. Bull., 2001, 50, 1998.
K. J. McNeil, J. A. DiCaprio, D. A. Walsh, R. F. Pratt, J. Am. Chem. Soc., 1980, 102, 1859.
P. V. Ivanov, N. I. Gel’perin, V. V. Kireev, Vysokomol. Soedin., Ser. A, 1985, 27, 1041 [Polym. Sci. USSR, Ser. A (Engl. Transl.), 1985, 27].
P. V. Ivanov, V. I. Maslova, N. M. Buzyreva, N. G. Mazhorova, D. N. Golubykh, L. V. Kostikova, A. S. Mozzhukhin, E. A. Chernyshev, Zh. Vsesoyuz. Khim. o-va im. D. I. Mendeleeva, 1998, 42, No. 6, 87 [Mendeleev Chem. J. (Engl. Transl.), 1998, 42, No. 6].
A. J. Gordon, R. A. Ford, The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References, 1973, Wiley, New York, 560 pp.
S. M. Walas, Phase Equilibria in Chemical Engineering, Butter Worth Publishers, London, 1985, 664 pp.
N. G. Mazhorova, P. V. Ivanov, Vestn. MITKhT [Bulletin of Moscow Institute of Fine Chemical Technology], 2013, 8, No. 5, 55 (in Russian).
T. I. Sunekants, G. A. Uvarova, O. V. Utkin, V. V. Severnyi, N. V. Varlamova, V. S. Kolobkov, N. V. Pavlova, T. L. Krasnova, E. A. Chernyshev, Zh. Prikl. Khim., 1985, 58, 341 [J. Appl. Chem. USSR (Engl. Transl.), 1985, 58].
P. V. Ivanov, Doct. Sci. (Chem.) Dissertation, M. V. Lomonosov Moscow State Academy of Fine Chemical Technologies, Moscow, 1998, 250 pp. (in Russian).
A. A. Fredenslung, R. L. Jones, J. M. Prausnits, AIChE J., 1975, 21, 1086.
A. A. Fredenslung, J. Gmehling, D. Russmussen, Vapour-Liquid Equilibria, Elsever, 1977, 392.
V. B. Kogan, S. K. Ogorodnikov, V. V. Kafarov, Spravochnik po rastvorimosti, 2, Troinye mnogokomponentnye sistemy [Reference Book on Solubility. Vol. 2. Ternary Multicomponent Systems], Izd. AN SSSR, Moscow, 1963, 946 pp. (in Russian).
K. A. Smith, J. Org. Chem., 1986, 51, 3827.
H. D. Cogan, Setterston Chemical and Eng. News, 1946, 24, 2499.
N. G. Mazhorova, Cand Sci. M. V. Lomonosov Moscow State University of Fine Chemical Technologies, Moscow, 2015, 133 pp. (in Russian).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are grateful to L. V. Kostikova (Moscow State Institute of Fine Chemical Technology) for calculations of the phase equilibrium RnSiCl4−n—Me2O—H2O by the UNIFAC method and to Yu. A. Pisarenko (Moscow State Institute of Fine Chemical Technology) for the mathematical simulation of phase equilibrium RnSi(OR′)4−n—Me2O—H2O on the basis of the NRTL equation.
On the occasion of the 65th anniversary of the foundation of A. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences.
Published in Russian in Izvestiya AkademiiNauk. Seriya Khimicheskaya, No. 6, pp. 1061–1071, June, 2020.
Rights and permissions
About this article
Cite this article
Ivanov, P.V., Mazhorova, N.G. Comparative analysis of phase diagrams of organochlorosilane/organoalkoxysilane—solvent—water systems. Russ Chem Bull 69, 1061–1071 (2020). https://doi.org/10.1007/s11172-020-2867-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2867-7