Abstract
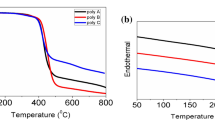
Novel donor–acceptor (D-A) conjugated polymers containing phenothiazine and diketopyrrolopyrrole derivatives were successfully synthesized via direct arylation polymerization using the palladium catalyst system. The C-N coupling reaction was performed for the synthesis of 4-(10H-phenothiazin-10-yl)-N,N-diphenylaniline (PDA) and 10-(pyren-1-yl)-10H-phenothiazine (PYP) as donor monomers. The D-A conjugated polymers synthesized by polymerization of PDA/PY with diketopyrrolopyrrole have been characterized via GPC, 1H NMR, FTIR, XRD, DSC, PL and UV-Vis spectroscopy, and used for fabrication of organic field effect transistors.

The novel D-A conjugated polymers containing phenothiazine derivatives and diketopyrrolopyrrole were successfully synthesized via direct (hetero)arylation polymerization. The obtained conjugated polymers have been applied for the fabrication of organic filed effect transistors. The conjugated polymers showed a hole mobility of 10−5 cm2/Vs in top contact OFET devices.










Similar content being viewed by others
References
Li Y (2012) Molecular Design of Photovoltaic Materials for polymer solar cells: toward suitable electronic energy levels and broad absorption. Acc Chem Res 45:723–733
Nguyen HT, Nguyen TT, Nguyen L-TT, Le TV, Nguyen VQ, Nguyen TA, Luu AT (2015) Synthesis and characterization of three-arm star-shaped conjugated poly(3-hexylthiophene)s: impact of the core structure on optical properties. Polym Int 64:1649–1659
Li S, Ye L, Zhao W, Zhang S, Mukherjee S, Ade H, Hou J (2016) Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells. Adv Mater 28:9423–9429
Kim JY, Lee K, Coates NE, Moses D, Nguyen T-Q, Dante M, Heeger AJ (2007) Efficient tandem polymer solar cells fabricated by all-solution processing. Science 317:222–225
Zhao J, Li Y, Yang G, Jiang K, Lin H, Ade H, Ma W, Yan H (2016) Efficient organic solar cells processed from hydrocarbon solvents. Nat Energy 1:15027
Kim BG, Ma X, Chen C, Ie Y, Coir EW, Hashemi H, Aso Y, Green PF, Kieffer J, Kim J (2013) Energy level modulation of HOMO, LUMO, and band-gap in conjugated polymers for organic photovoltaic applications. Adv Funct Mater 23:439–445
Jhuo HJ, Yeh PN, Liao SH, Li YL, Cheng YS, Chen SA (2014) Review on the recent Progress in low band gap conjugated polymers for bulk hetero-junction polymer solar cells. J Chin Chem Soc 61:115–126
Li H, Earmme T, Subramaniyan S, Jenekhe SA (2015) Bis (naphthalene imide) diphenylanthrazolines: a new class of electron acceptors for efficient nonfullerene organic solar cells and applicable to multiple donor polymers. Adv Energy Mater 5:1402041
Li H, Hwang YJ, Courtright BA, Eberle FN, Subramaniyan S, Jenekhe SA (2015) Fine-tuning the 3D structure of nonfullerene Electron acceptors toward high-performance polymer solar cells. Adv Mater 27:3266–3272
Wienk MM, Turbiez M, Gilot J, Janssen RA (2008) Narrow-bandgap diketo-pyrrolo-pyrrole polymer solar cells: the effect of processing on the performance. Adv Mater 20:2556–2560
Pan H, Wu Y, Li Y, Liu P, Ong BS, Zhu S, Xu G (2007) Benzodithiophene copolymer—a low-temperature, solution-processed high-performance semiconductor for thin-film transistors. Adv Funct Mater 17:3574–3579
Li Y, Wu Y, Liu P, Birau M, Pan H, Ong BS (2006) Poly (2, 5-bis (2-thienyl)-3, 6-dialkylthieno [3, 2-b] thiophene) s—high-mobility semiconductors for thin-film transistors. Adv Mater 18:3029–3032
Lim ZB, Xue B, Bomma S, Li H, Sun S, Lam YM, Belcher WJ, Dastoor PC, Grimsdale AC (2011) New moderate bandgap polymers containing alkoxysubstituted-benzo [c][1, 2, 5] thiadiazole and thiophene-based units. J Polym Sci Part A: Polym Chem 49:4387–4397
Li Y, Sonar P, Murphy L, Hong W (2013) High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ Sci 6:1684–1710
Qu S, Tian H (2012) Diketopyrrolopyrrole (DPP)-based materials for organic photovoltaics. Chem Commun 48:3039–3051
Lee J, Park HJ, Joo JM, Hwang D-H (2019) Synthesis and characterization of DPP-based conjugated polymers via Dehydrogenative direct Alkenylation Polycondensation. Macromol Res 27:115–118
Li W, Hendriks KH, Wienk MM, Janssen RA (2015) Diketopyrrolopyrrole polymers for organic solar cells. Acc Chem Res 49:78–85
Zhao C, Guo Y, Zhang Y, Yan N, You S, Li W (2019) Diketopyrrolopyrrole-based conjugated materials for non-fullerene organic solar cells. J Mater Chem A 7:10174–10199
Sun P, Li X, Wang Y, Shan H, Xu J, Liu C, Zhang C, Chen F, Xu Z, Z-k C (2018) Diketopyrrolopyrrole-based acceptors with multi-arms for organic solar cells. RSC Adv 8:25031–25039
Bi S, Li Y, He Z, Ouyang Z, Guo Q, Jiang C (2019) Self-assembly diketopyrrolopyrrole-based materials and polymer blend with enhanced crystal alignment and property for organic field-effect transistors. Org Electron 65:96–99
Jiramitmongkon K, Chotsuwan C, Asawapirom U, Hirunsit P (2019) Cyclopentadithiophene and Diketo-pyrrolo-pyrrole fused rigid copolymer for high optical contrast electrochromic polymer. J Polym Res 27:17
Bi S, He Z, Chen J, Li D (2015) Solution-grown small-molecule organic semiconductor with enhanced crystal alignment and areal coverage for organic thin film transistors. AIP Adv 5:077170
Negru OI, Grigoras M (2019) Synthesis and properties of copolyarylenes containing indolo[3,2-b]carbazole moieties in the backbone. J Polym Res 26:30
Ramachandran M, Raj MR, Azeez UHA, Sorrentino A, Anandan S, Ashokkumar M (2020) Synthesis of random copolymer using Zig-Zag Naphthodithiophene for bulk Heterojunction polymer solar cell applications. J Polym Res 27:171
Kong X, Kulkarni AP, Jenekhe SA (2003) Phenothiazine-based conjugated polymers: synthesis, electrochemistry, and light-emitting properties. Macromolecules 36:8992–8999
Lai RY, Fabrizio EF, Lu L, Jenekhe SA, Bard AJ (2001) Synthesis, cyclic voltammetric studies, and electrogenerated chemiluminescence of a new donor acceptor molecule: 3, 7-[Bis [4-phenyl-2-quinolyl]]-10-methylphenothiazine. J Am Chem Soc 123:9112–9118
Lai RY, Kong X, Jenekhe SA, Bard AJ (2003) Synthesis, cyclic voltammetric studies, and electrogenerated chemiluminescence of a new phenylquinoline-biphenothiazine donor− acceptor molecule. J Am Chem Soc 125:12631–12639
Krämer CS, Zeitler K, Müller TJ (2000) Synthesis of functionalized ethynylphenothiazine fluorophores. Org Lett 2:3723–3726
Krämer C, Zeitler K, Müller T (2001) First synthesis and electronic properties of (hetero) aryl bridged and directly linked redox active phenothiazinyl dyads and triads. Tetrahedron Lett 42:8619–8624
Ruiz-Castillo P, Buchwald SL (2016) Applications of palladium-catalyzed C–N cross-coupling reactions. Chem Rev 116:12564–12649
Bredas J, Heeger A (1990) Theoretical investigation of gas-phase torsion potentials along conjugated polymer backbones: polyacetylene, polydiacetylene, and polythiophene. Macromolecules 23:1150–1156
Chen T-A, Wu X, Rieke RD (1995) Regiocontrolled synthesis of poly (3-alkylthiophenes) mediated by Rieke zinc: their characterization and solid-state properties. J Am Chem Soc 117:233–244
Yuan Y, Zhang J, Sun J, Hu J, Zhang T, Duan Y (2011) Polymorphism and structural transition around 54 C in regioregular poly (3-hexylthiophene) with high crystallinity as revealed by infrared spectroscopy. Macromolecules 44:9341–9350
Dag S, Wang L-W (2010) Packing structure of poly (3-hexylthiophene) crystal: ab initio and molecular dynamics studies. J Phys Chem B 114:5997–6000
Nguyen TA, Luu AT, Nguyen TH, Le NM, Tran HM, Nguyen L-TT, Lee JY, Nguyen HT (2017) Thiacalix[3]Triazine-centered regioregular poly(3-hexylthiophene) star: synthesis, structure and anion binding. J Polym Res 24:180
Zhang G, Guo J, Zhang J, Li P, Ma J, Wang X, Lu H, Qiu L (2015) A phthalimide-and diketopyrrolopyrrole-based a 1–π–a 2 conjugated polymer for high-performance organic thin-film transistors. Polym Chem 6:418–425
Horowitz G (1998) Organic field-effect transistors. Adv Mater 10:365–377
Urien M, Wantz G, Cloutet E, Hirsch L, Tardy P, Vignau L, Cramail H, Parneix J-P (2007) Field-effect transistors based on poly (3-hexylthiophene): effect of impurities. Org Electron 8:727–734
Tu G, Bilge A, Adamczyk S, Forster M, Heiderhoff R, Balk LJ, Mühlbacher D, Morana M, Koppe M, Scharber MC, Choulis SA, Brabec CJ, Scherf U (2007) The influence of Interchain branches on solid state packing, hole mobility and photovoltaic properties of poly(3-hexylthiophene) (P3HT). Macromol Rapid Commun 28:1781–1785
Nawaz A, Cruz-Cruz I, Rodrigues R, Hümmelgen IA (2015) Performance enhancement of poly(3-hexylthiophene-2,5-diyl) based field effect transistors through surfactant treatment of the poly(vinyl alcohol) gate insulator surface. PCCP 17:26530–26534
de Col C, Nawaz A, Cruz-Cruz I, Kumar A, Kumar A, Hümmelgen IA (2015) Poly(vinyl alcohol) gate dielectric surface treatment with vitamin C for poly(3-hexylthiophene-2,5-diyl) based field effect transistors performance improvement. Org Electron 17:22–27
He Z, Zhang Z, Bi S (2019) Long-range crystal alignment with polymer additive for organic thin film transistors. J Polym Res 26:173
He Z, Zhang Z, Bi S, Asare-Yeboah K, Chen J (2020) Ultra-low misorientation angle in small-molecule semiconductor/polyethylene oxide blends for organic thin film transistors. J Polym Res 27:75
He Z, Zhang Z, Asare-Yeboah K, Bi S (2019) Poly(α-methylstyrene) polymer and small-molecule semiconductor blend with reduced crystal misorientation for organic thin film transistors. J Mater Sci Mater Electron 30:14335–14343
He Z, Zhang Z, Bi S, Chen J, Li D (2020) Conjugated polymer controlled morphology and charge transport of small-molecule organic semiconductors. Sci Rep 10:4344
Acknowledgements
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number B2019-20-12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 239 kb)
Rights and permissions
About this article
Cite this article
Truong, N.T.T., Nguyen, L.T., Mai, H.L.T. et al. Phenothiazine derivatives, diketopyrrolopyrrole-based conjugated polymers: synthesis, optical and organic field effect transistor properties. J Polym Res 27, 223 (2020). https://doi.org/10.1007/s10965-020-02199-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02199-x