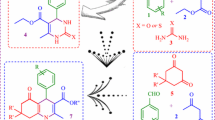
Abstract
Spinel-type oxide MgMnO3 and perovskite-type oxide SrMnO3 have been synthesized by two-step procedures comprising the hydrothermal and solid-state methods. The as-prepared catalysts were characterized by X-ray powder diffraction, Fourier transform infrared spectroscopy, thermogravimetric analysis and the morphologies of the synthesized materials were figured out by field emission scanning electron microscopy. The catalytic performance of synthesized materials were investigated by synthesizing the polyhydroquinolines, which was used Design-Expert software and response surface methodology (central composite design) for optimization of reaction efficiently. The comparison study illustrates that MgMnO3 has better catalytic performance than SrMnO3 and optimized condition for the synthesis of polyhydroquinoline (which is produced by the reaction of ethyl acetoacetate, benzaldehyde, dimedone and ammonium acetate) was found to be 8 mg of MgMnO3 as the catalyst, 33 min for the reaction time and 100 °C for the reaction temperature. This condition was used for the synthesis of other polyhydroquinoline derivatives.









Similar content being viewed by others
References
Arslan M, Faydali C, Zengin M, Küçükislamoğlu M, Demirhan H (2009) An efficient one pot synthesis of 1, 4-dihydropyridines using alumina sulfuric acid (ASA) catalyst. Turk J Chem 33(6):769–774
Beyranvand M, Gholizadeh A (2020) Structural, magnetic, elastic, and dielectric properties of Mn0.3−xCdxCu0.2Zn0.5Fe2O4 nanoparticles. J Mater Sci Mater Electron 31(7):5124–5140
Bhattacharya A, May S, Te Velthuis S, Warusawithana M, Zhai X, Jiang B, Zuo J-M, Fitzsimmons M, Bader S, Eckstein J (2008) Metal-insulator transition and its relation to magnetic structure in (LaMnO3)2n/(SrMnO3)n superlattices. Phys Rev Lett 100:203–257
Borhade A, Tope D, Gare G, Dabhade G (2017) One pot four-component synthesis of novel substituted 2-phenyl-4 (3H) quinazolinones using recyclable nanocrystalline CuMnO3 catalyst. J Korean Chem Soc 61:157–162
Buhler FR, Kiowski WJ (1987) Calcium antagonists in hypertension. Hypertension 5:S3–S10
Das B, Ravikanth B, Ramu R, Rao BV (2006) An efficient one-pot synthesis of polyhydroquinolines at room temperature using HY-zeolite. Chem Pharm Bull 54:1044–1045
Donelson JL, Gibbs RA, De SK (2006) An efficient one-pot synthesis of polyhydroquinoline derivatives through the Hantzsch four component condensation. J Mol Catal A Chem 256:309–311
Esmaili L, Gholizadeh A (2020) The effect of Nd and Zr co-substitution on structural, magnetic and photocatalytic properties of Bi1−xNdxFe1−xZrxO3 nanoparticles. Mater Sci Semicond Process 118:105179
Geusic J, Kurtz S, Van Uitert L, Wemple S (1964) Electro‐optic properties of some ABO3 perovskites in the paraelectric phase. Appl Phys Lett 4:141
Gholizadeh A (2017) A comparative study of physical properties in Fe3O4 nanoparticles prepared by coprecipitation and citrate methods. J Am Ceram Soc 100(8):3577–3588
Gholizadeh A (2018) A comparative study of the physical properties of Cu–Zn ferrites annealed under different atmospheres and temperatures: magnetic enhancement of Cu0.5Zn0.5Fe2O4 nanoparticles by a reducing atmosphere. J Magn Magn Mater 452:389–397
Gholizadeh A (2019) The effects of A/B-site substitution on structural, redox and catalytic properties of lanthanum ferrite nanoparticles. J Mater Res Technol 8(1):457–466
Gholizadeh A, Beyranvand M (2020) Investigation on the structural, magnetic, dielectric and impedance analysis of Mg0.3−xBaxCu0.2Zn0.5Fe2O4 nanoparticles. Phys B Condens Matter 584:412079
Gholizadeh A, Jafari E (2017) Effects of sintering atmosphere and temperature on structural and magnetic properties of Ni–Cu–Zn ferrite nano-particles: magnetic enhancement by a reducing atmosphere. J Magn Magn Mater 422:328–336
Gholizadeh A, Malekzadeh A (2017) Structural and redox features of La0.7Bi0.3Mn1−xCoxO3 nanoperovskites for ethane combustion and CO oxidation. Int J Appl Ceram Technol 14(3):404–412
Gholizadeh A, Yousefi H, Malekzadeh A, Pourarian F (2016) Calcium and strontium substituted lanthanum manganite–cobaltite [La1−x(Ca, Sr)xMn0.5Co0.5O3] nanocatalysts for low temperature CO oxidation. Ceram Int 42(10):12055–12063
Gholizadeh A, Malekzadeh A, Pourarian F (2018) Rapid and efficient synthesis of reduced graphene oxide nano-sheets using CO ambient atmosphere as a reducing agent. J Mater Sci: Mater Electron 29:19402–19412
Irandoust R, Gholizadeh A (2020) A comparative study of the effect of the non-magnetic and magnetic trivalent rare-earth ion substitutions on bismuth ferrite properties: correlation between the crystal structure and physical properties. Solid State Sci 101:106142
Karade NN, Budhewar VH, Shinde SV, Jadhav WN (2007) L-proline as an efficient organo-catalyst for the synthesis of polyhydroquinoline via multicomponent Hantzsch reaction. Lett Org Chem 4:16–19
Kassaee M, Masrouri H, Movahedi F (2010) ZnO-nanoparticle-promoted synthesis of polyhydroquinoline derivatives via multicomponent Hantzsch reaction. Chem Chem Mon 141:317–322
Kelley HJ (1962) Method of gradients. Math Sci Eng 5:205–254
Khabazzadeh H, Kermani ET, Afzali D, Amiri A, Jalaladini A (2012) Efficient one-pot synthesis of polyhydroquinoline derivatives using Cs2.5H0.5PW12O40 as a heterogeneous and reusable catalyst in molten salt media. Arab J Chem 5:167–172
Khedri H, Gholizadeh A (2019) Experimental comparison of structural, magnetic and elastic properties of M0.3Cu0.2Zn0.5Fe2O4 (M = Cu, Mn, Fe Co, Ni, Mg) nanoparticles. Appl Phys Mater Sci Process 125:709
Maheswara M, Siddaiah V, Damu GLV, Rao CV (2006) An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditions. Arkivoc 2:201–206
Mahmoudi S, Gholizadeh A (2018) Effect of non-magnetic ions substitution on the structure and magnetic properties of Y3−xSrxFe5−xZrxO12 nanoparticles. J Magn Magn Mater 456:46–55
Markhele V, Sadaphal S, Shingare M (2007) An efficient one-pot synthesis of polyhydroquinolines at room temperature using MCM-41 catalyst under solvent-free conditions. Bull Catal Soc India 6:125–131
Mondal S, Patra BC, Bhaumik A (2017) One-pot synthesis of polyhydroquinoline derivatives through organic-solid-acid-catalyzed Hantzsch condensation reaction. ChemCatChem 9:1469–1475
Nasr-Esfahani M, Hoseini SJ, Montazerozohori M, Mehrabi R, Nasrabadi H (2014) Magnetic Fe3O4 nanoparticles: efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and Hantzsch 1,4-dihydropyridines under solvent-free conditions. J Mol Catal A Chem 382:99–105
Njagi EC, Genuino HC, Kingondu CK, Dharmarathna S, Suib SL (2012) Electro-optic properties of some ABO3 perovskites in the paraelectric phase. Appl Catal A Gen 421–422:154–160
Rajini A, Nookaraju M, Reddy IAK, Narayanan V (2014) Vanadium dodecylamino phosphate: a novel efficient catalyst for synthesis of polyhydroquinolines. Chem Pap 68:170–179
Rao GD, Nagakalyan S, Prasad G (2017) Solvent-free synthesis of polyhydroquinoline derivatives employing mesoporous vanadium ion doped titania nanoparticles as a robust heterogeneous catalyst via the Hantzsch reaction. RSC Adv 7:3611–3616
Rostamnia S, Pourhassan F (2013) The SBA-15/SO3H nanoreactor as a highly efficient and reusable catalyst for diketene-based, four-component synthesis of polyhydroquinolines and dihydropyridines under neat conditions. Chin Chem Lett 24:401–403
Safaei-Ghomi J, Ghasemzadeh M (2011) Nanocrystalline copper(II) oxide-catalyzed one-pot four component synthesis of polyhydroquinoline derivativesunder solvent-free conditions. J Nanostruct 1:243–248
Saha M, Pal AK (2011) Palladium (0) nanoparticles: an efficient catalyst for the one-pot synthesis of polyhydroquinolines. Tetrahedron Lett 52:4872–4877
Sapkal SB, Shelke KF, Shingate BB, Shingare MS (2009) Nickel nanoparticle-catalyzed facile and efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions. Tetrahedron Lett 50:1754–1756
Shamgani N, Gholizadeh A (2019) Structural, magnetic and elastic properties of Mn0.3−xMgxCu0.2Zn0.5Fe3O4 nanoparticles. Ceram Int 45:239–246
Soleimani F, Salehi M, Gholizadeh A (2019) Comparison of visible light photocatalytic degradation of different pollutants by (Zn, Mg)xCu1-xBi2O4 nanoparticles. Ceram Int 45(7):8926–8939
Tabrizian E, Amoozadeh A (2016) A new type of SO3H-functionalized magnetic-titania as a robust magnetically-recoverable solid acid nanocatalyst for multi-component reactions. RSC Adv 6:96606–96615
Tauler R, Walczak B, Brown SD (2009) Comprehensive chemometrics: chemical and biochemical data analysis, 1st edn. Elsevier, Amsterdam
Tekale SU, Pagore VP, Kauthale SS, Pawar RP (2014) La2O3/TFE: an efficient system for room temperature synthesis of Hantzsch polyhydroquinolines. Chin Chem Lett 25:1149–1152
Theivasanthi T, Alagar M (2011) An insight analysis of nano sized powder of jackfruit seed. Nano Biomed Eng. https://doi.org/10.5101/nbe.v3i3.p163-168
Thota S, Singh K, Prasad B, Kumar J, Simon C, Prellier W (2012) Formation mechanism, optical and magneto-dielectric studies of new cubic spinel MgMnO3. AIP Adv 2:1032–1040
Wang Q, Xu J, Wang X, Liu B, Hou X, Yu G, Wang P, Chen D, Shen G (2014) Core–shell CuCo2O4@MnO2 nanowires on carbon fabrics as high-performance materials for flexible, all-solid-state, electrochemical capacitors. ChemElectroChem 1:559–564
Yin B, Zhang S, Jiao Y, Liu Y, Qu F, Wu X (2014) Facile synthesis of ultralong MnO2 nanowires as high performance supercapacitor electrodes and photocatalysts with enhanced photocatalytic activities. CrystEngCommun 16:9999–10005
Ziarani GM, Badiei AR, Khaniania Y, Haddadpour M (2010) One pot synthesis of polyhydroquinolines catalyzed by sulfonic acid functionalized SBA-15 as a new nanoporous acid catalyst under solvent free conditions. Iran J Chem Chem Eng 29:1–10
Acknowledgements
This work was financially supported the by Chemistry Department, Semnan University of Iran, and the authors gratefully thank their cooperation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Soleimani, F., Salehi, M. & Gholizadeh, A. Comparing Catalytic Activity of MgMnO3 and SrMnO3 Nanocatalyst for Synthesis of Polyhydroquinoline and New Derivatives via Hantzsch Reaction. Iran J Sci Technol Trans Sci 44, 1011–1023 (2020). https://doi.org/10.1007/s40995-020-00920-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-020-00920-5