Abstract
Purpose
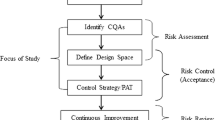
The purpose of the study was to develop a model to predict the critical quality attribute (CQA) of tablets during continuous and batch manufacturing using only critical material attributes (CMAs).
Methods
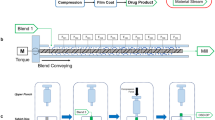
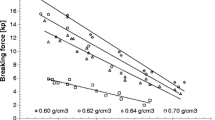
Experiments were performed using ethenzamide as the active pharmaceutical ingredient processed with batch and continuous high-shear granulators. The disintegration time of tablets was defined as the CQA, and the particle-size distribution of granules and tablet hardness were defined as the CMAs. We first investigated the influence of granulation conditions on particle-size distribution during batch and continuous granulation. We then proceeded to construct the CQA estimation model by producing tables using batch and continuous granulation.
Results
The results indicated the similarity of the granulation mechanisms, as observed by the bimodality of the distributions and the significant causal factors. Principal component analysis revealed that the CQA was influenced strongly by the particle-size distribution and that the CMA–CQA correlations were similar for both processes. Finally, a model based on partial least-squares regression could be developed that could reasonably estimate the CQA using CMAs without involving any process parameters.
Conclusion
This approach of using process-independent CQA prediction could enable flexible switching between batch and continuous manufacturing during a product life cycle, thus offering new possibilities for efficient life cycle management.






Similar content being viewed by others
References
Leuenberger H. New trends in the production of pharmaceutical granules: the classical batch concept and the problem of scale-up. Eur J Pharm Biopharm. 2001;52(3):279–88. https://doi.org/10.1016/S0939-6411(01)00200-4.
Aikawa S, Fujita N, Myojo H, Hayashi T, Tanino T. Scale-up studies on high shear wet granulation process from mini-scale to commercial scale. Chem Pharm Bull (Tokyo). 2008;56(10):1431–5. https://doi.org/10.1248/cpb.56.1431.
Campbell GA, Clancy DJ, Zhang JX, Gupta MK, Oh CK. Closing the gap in series scale up of high shear wet granulation process using impeller power and blade design. Powder Technol. 2011;205(1–3):184–92. https://doi.org/10.1016/j.powtec.2010.09.009.
Luo G, Xu B, Zhang Y, Cui X, Li J, Shi X, et al. Scale-up of a high shear wet granulation process using a nucleation regime map approach. Particuology. 2017;31:87–94. https://doi.org/10.1016/j.partic.2016.04.007.
GEA. Continuous granulation: ConsiGma™. GEA. 2020. https://www.gea.com/en/applications/pharma/solid-dosage/solid-dosage_continuous-granulation.jsp. Accessed 10 May 2020.
Powrex Corporation. Continuous granulation: MiGRA. Powrex Corporation. 2019. https://www.powrex.co.jp/migra-en. Accessed 10 May 2019.
Allison G, Cain YT, Cooney C, Garcia T, Bizjak TG, Holte O, et al. Regulatory and quality considerations for continuous manufacturing May 20–21, 2014 Continuous Manufacturing Symposium. J Pharm Sci. 2015;104:803–12. https://doi.org/10.1002/jps.24324.
Singh R, Román-Ospino AD, Romañach RJ, Ierapetritou M, Ramachandran R. Real time monitoring of powder blend bulk density for coupled feed-forward/feed-back control of a continuous direct compaction tablet manufacturing process. Int J Pharm. 2015;495(1):612–25. https://doi.org/10.1016/j.ijpharm.2015.09.029.
Toson P, Siegmann E, Trogrlic M, Kureck H, Khinast J, Jajcevic D, et al. Detailed modeling and process design of an advanced continuous powder mixer. Int J Pharm. 2018;552(1–2):288–300. https://doi.org/10.1016/j.ijpharm.2018.09.032.
Lee KT, Ingram A, Rowson NA. Comparison of granule properties produced using twin screw extruder and high shear mixer: a step towards understanding the mechanism of twin-screw wet granulation. Powder Technol. 2013;238:91–8. https://doi.org/10.1016/j.powtec.2012.05.031.
Beer P, Wilson D, Huang Z, De Matas M. Transfer from high-shear batch to continuous twin screw wet granulation: a case study in understanding the relationship between process parameters and product quality attributes. J Pharm Sci. 2014;103(10):3075–82. https://doi.org/10.1002/jps.24078.
Järvinen MA, Paavola M, Poutiainen S, Itkonen P, Pasanen V, Uljas K, et al. Comparison of a continuous ring layer wet granulation process with batch high shear and fluidized bed granulation processes. Powder Technol. 2015;275:113–20. https://doi.org/10.1016/j.powtec.2015.01.071.
Matsunami K, Nagato T, Hasegawa K, Sugiyama H. A large-scale experimental comparison of batch and continuous technologies in pharmaceutical tablet manufacturing using ethenzamide. Int J Pharm. 2019;559:210–9. https://doi.org/10.1016/j.ijpharm.2019.01.028.
Matsunami K, Nagato T, Hasegawa K, Sugiyama H. Determining key parameters of continuous wet granulation for tablet quality and productivity: a case in ethenzamide. Int J Pharm. 2020;579:119160. https://doi.org/10.1016/j.ijpharm.2020.119160.
National Institute of Health Sciences (NIHS). The Japanese Pharmacopoeia, Seventeenth Edition (JP17). NIHS. 2019. http://jpdb.nihs.go.jp/jp17e/. Accessed 4 Dec 2019.
Acknowledgments
The authors acknowledge Mr. Kazuhiro Kotaka at Powrex Corporation for conducting the experiments in batch and continuous high-shear granulation. The authors also thank the members of the Focus Group of the Academy of Pharmaceutical Science and Technology for providing great support to this research and also Mr. Kensaku Matsunami at the University of Tokyo for assistance with the literature survey.
Funding
The research was partly funded by Powrex Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work presents a model that can predict a critical quality attribute (CQA) of tablets prepared by both batch and continuous manufacturing based only on their critical material attributes (CMAs), towards flexible technology switching.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Arai, H., Nagato, T., Koide, T. et al. Tablet Quality-Prediction Model Using Quality Material Attributes: Toward Flexible Switching Between Batch and Continuous Granulation. J Pharm Innov 16, 588–602 (2021). https://doi.org/10.1007/s12247-020-09466-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09466-w