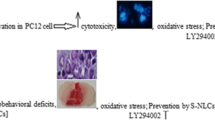
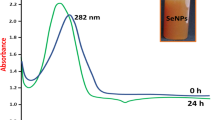
Abstract
Ischemic stroke is the leading cause of disability and death worldwide. Currently, the only proven treatment for ischemic stroke is restoring the cerebral blood supply. In addition, some of the tissue is damaged during the subsequent reperfusion because of the overproduction of reactive oxygen species (ROS). Furthermore, antioxidant therapies have shown promise in preclinical studies for the treatment of ischemia-reperfusion injury. However, their therapeutic efficacy has been limited because of their low bioavailability in brain. To resolve this issue, we synthesized ROS-responsive, fan-shaped dendrimer nanoparticles (NPs) and conjugated them with a blood-brain barrier (BBB)-targeting peptide, COG1410, and salvianic acid A (SA), which is an effective antioxidant in ischemic stroke. The BBB targeting peptide acts as a ligand of the nanocarrier system and penetrates the BBB through the endocytosis of the ligand receptor. The results showed that T-SA-NPs not only target and accumulate in the infarct area, they also reduce over 2 times of the infarct area and reverse the behavioral deficits in MCAO mice, which illustrates that these NPs have an effective therapeutic effect on the ischemic stroke. In addition, these NPs had no toxicity in any organs of the body. Importantly, the present study provides an alternative strategy for delivering antioxidants to the brain and achieving targeted therapy of ischemic stroke.

Similar content being viewed by others
References
Donnan, G. A.; Fisher, M.; Macleod, M.; Davis, S. M. Stroke. Lancet2008, 371, 1612–1623.
Campbell, B. C. V.; Ma, H.; Ringleb, P. A.; Parsons, M. W.; Churilov, L.; Bendszus, M.; Levi, C. R.; Hsu, C.; Kleinig, T. J.; Fatar, M. et al. Extending thrombolysis to 4-5-9 h and wake-up stroke using perfusion imaging: A systematic review and meta-analysis of individual patient data. Lancet2019, 394, 139–147.
Coutts, S. B.; Menon, B. K. Late thrombolysis for stroke works, but how do we do it? Lancet2019, 394, 97–98.
Chouchani, E. T.; Pell, V. R.; Gaude, E.; Aksentijević, D.; Sundier, S. Y.; Robb, E. L.; Logan, A.; Nadtochiy, S. M.; Ord, E. N. J.; Smith, A. C. et al. Ischemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature2014, 515, 431–435.
Yamato, M.; Egashira, T.; Utsumi, H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic. Biol. Med.2003, 35, 1619–1631.
Kim, C. K.; Kim, T.; Choi, I. Y.; Soh, M.; Kim, D.; Kim, Y. J.; Jang, H.; Yang, H. S.; Kim, J. Y.; Park, H. K. et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem., Int. Ed. Engl.2012, 51, 11039–11043.
Parikh, N. S.; Elkind, M. S. V. Divergent effects of lipids on stroke. Nat. Med.2019, 25, 543–544.
Liu, X. J.; Yan, L.; Xue, F. Z. The associations of lipids and lipid ratios with stroke: A prospective cohort study. J. Clin. Hypertens.2019, 21, 127–135.
Holmes, M. V.; Millwood, I. Y.; Kartsonaki, C.; Hill, M. R.; Bennett, D. A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R. Y. et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J. Am. Coll. Cardiol.2018, 71, 620–632.
Ding, W.; Hudson, L. G.; Liu, K. J. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol. Cell. Biochem.2005, 279, 105–112.
Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z. H.; Hui, J.; Graham, B. H.; Quintana, A. et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell2015, 160, 177–190.
Melo, A.; Monteiro, L.; Lima, R. M. F.; De Oliveira, D. M.; De Cerqueira, M. D.; El-Bachá, R. S. Oxidative stress in neurodegenerative diseases: Mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longev.2011, 2011, 467180.
Reed, T. T. Lipid peroxidation and neurodegenerative disease. Free Radic Biol. Med.2011, 51, 1302–1319.
Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E. N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta2014, 436, 332–347.
Fan, Y.; Luo, Q. P.; Wei, J. J.; Lin, R. H.; Lin, L. L.; Li, Y. K.; Chen, Z. R.; Lin, W.; Chen, Q. Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Res.2018, 1679, 125–133.
Chang, Y.; Hsieh, C. Y.; Peng, Z. A.; Yen, T. L.; Hsiao, G.; Chou, D. S.; Chen, C. M.; Sheu, J. R. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J. Biomed. Sci.2009, 16, 9.
Yan, R. Y.; Wang, S. J.; Yao, G. T.; Liu, Z. G.; Xiao, N. The protective effect and its mechanism of 3-n-butylphthalide pretreatment on cerebral ischemia reperfusion injury in rats. Eur. Rev. Med. Pharmacol. Sci.2017, 21, 5275–5282.
Yang, Y. J.; Su, Y. J.; Wang, D. T.; Chen, Y. H.; Wu, T.; Li, G.; Sun, X. G.; Cui, L. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signaling. Oxid. Med. Cell. Longev.2013, 2013, 351895.
Song, W.; Pu, J.; He, B. Tanshinol protects human umbilical vein endothelial cells against hydrogen peroxide-induced apoptosis. Mol. Med. Rep.2014, 10, 2764–2770.
Chong, C. M.; Zhou, Z. Y.; Razmovski-Naumovski, V.; Cui, G. Z.; Zhang, L. Q.; Sa, F.; Hoi, P. M.; Chan, K.; Lee, S. M. Y. Danshensu protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Neurosci. Lett., 2013, 543, 121–125.
Wei, Y.; Zhang, L.; Yu, Z. P.; Lin, K. S.; Yang, S. F.; Dai, L.; Liu, J. F.; Mao, L. K.; Yuan, F.; Gao, Y. X. Enhanced stability, structural characterization and simulated gastrointestinal digestion of coenzyme Q10 loaded ternary nanoparticles. Food Hydr.2019, 94, 333–344.
Liu, C. Z.; Zhang, S. Z.; McClements, D. J.; Wang, D. F.; Xu, Y. Design of Astaxanthin-loaded core-shell nanoparticles consisting of chitosan oligosaccharides and poly (lactic-co-glycolic acid): Enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem.2019, 67, 5113–5121.
Esposito, E.; Drechsler, M.; Puglia, C.; Cortesi, R. New strategies for the delivery of some natural anti-oxidants with therapeutic properties. Mini-Rev. Med. Chem.2019, 19, 1030–1039.
Chen, C. T.; Duan, Z. Q.; Yuan, Y.; Li, R. X.; Pang, L.; Liang, J. M.; Xu, X. C.; Wang, J. X. Peptide-22 and cyclic RGD functionalized liposomes for glioma targeting drug delivery overcoming BBB and BBTB. ACS Appl. Mater. Interfaces2017, 9, 5864–5873.
Yin, T. T.; Xie, W. J.; Sun, J.; Yang, L. C.; Liu, J. Penetratin peptide-functionalized gold nanostars: Enhanced BBB permeability and NIR photothermal treatment of Alzheimer’s disease using ultralow irradiance. ACS Appl. Mater. Interfaces2016, 8, 19291–19302.
Yang, J. T.; Kuo, Y. C.; Chen, I. Y.; Rajesh, R.; Lou, Y. I.; Hsu, J. P. Protection against neurodegeneration in the hippocampus using sialic acid- and 5-HT-moduline-conjugated lipopolymer nanoparticles. ACS Biomater. Sci. Eng.2019, 5, 1311–1320.
Malvajerd, S. S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M. S.; Javar, H. A.; Hamidi, M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: Preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci.2019, 10, 728–739.
Zhang, P.; Omaye, S. T. DNA strand breakage and oxygen tension: Effects of β-carotene, α-tocopherol and ascorbic acid. Food Chem. Toxicol.2001, 39, 239–246.
Ekladious, I.; Colson, Y. L.; Grinstaff, M. W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug. Discov.2019, 18, 273–294.
Mohammed, F.; Ke, W. D.; Mukerabigwi, J. F.; Japir A. A. W. M.; Ibrahim, A.; Wang, Y. H.; Zha, Z. S.; Lu, N. N.; Zhou, M.; Ge, Z. S. ROS-responsive polymeric nanocarriers with photoinduced exposure of cell-penetrating moieties for specific intracellular drug delivery. ACS Appl. Mater. Interfaces2019, 11, 31681–31692.
Xiang, J. J.; Liu, X.; Zhou, Z. X.; Zhu, D. C.; Zhou, Q.; Piao, Y.; Jiang, L. M.; Tang, J. B.; Liu, X. R.; Shen, Y. Q. Reactive oxygen species (ROS)-responsive charge-switchable nanocarriers for gene therapy of metastatic cancer. ACS Appl. Mater. Interfaces2018, 10, 43352–43362.
Zhang, M.; Song, C. C.; Su, S.; Du, F. S.; Li, Z. C. ROS-activated ratiometric fluorescent polymeric nanoparticles for self-reporting drug delivery. ACS Appl. Mater. Interfaces2018, 10, 7798–7810.
Qiao, Y. T.; Wan, J. Q.; Zhou, L. Q.; Ma, W.; Yang, Y. Y.; Luo, W. X.; Yu, Z. Q.; Wang, H. X. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wires Nanomed. Nanobi.2019, 11, e1527.
Kalhapure, R. S.; Renukuntla, J. Thermo- and pH dual responsive polymeric micelles and nanoparticles. Chem. Biol. Int.2018, 295, 20–37.
Xu, X. D.; Saw, P. E.; Tao, W.; Li, Y. J.; Ji, X. Y.; Bhasin, S.; Liu, Y. L.; Ayyash, D.; Rasmussen, J.; Huo, M. et al. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv. Mater.2017, 29, 1700141.
Zhang, X. Q.; Li, L. H.; Li, C. F.; Zheng, H.; Song, H. Y.; Xiong, F. L.; Qiu, T.; Yang, J. Cisplatin-crosslinked glutathione-sensitive micelles loaded with doxorubicin for combination and targeted therapy of tumors. Carbohydr. Polym.2017, 155, 407–415.
Sharma, A. K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A. K.; Gupta, U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Dis. Today2017, 22, 314–326.
Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M. C. I. M.; Gupta, U.; Greish, K.; Iyer, A. K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater.2016, 43, 14–29.
Lu, Y. P.; Han, S. P.; Zheng, H. Y.; Ma, R.; Ping, Y. T.; Zou, J. F.; Tang, H. X.; Zhang, Y. P.; Xu, X. L.; Li, F. Z. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int. J. Nanomedicine2018, 13, 5937–5952.
Gothwal, A.; Nakhate, K. T.; Alexander, A.; Ajazuddin; Gupta, U. Boosted memory and improved brain bioavailability of rivastigmine: Targeting effort to the brain using covalently tethered lower generation PAMAM dendrimers with lactoferrin. Mol. Pharmaceutics2018, 15, 4538–4549.
Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R. M. Targeting mitochondrial dysfunction and oxidative stress in activated microglia using dendrimer-based therapeutics. Theranostics2018, 8, 5529–5547.
Guo, X. L.; Kang, X. X.; Wang, Y. Q.; Zhang, X. J.; Li, C. J.; Liu, Y.; Du, L. B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater.2019, 84, 367–377.
Chiou, S. H.; Wu, W. T. Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials2004, 25, 197–204.
Guo, X. L.; Kang, X. X.; Wang, Y. Q.; Zhang, X. J.; Li, C. J.; Liu, Y.; Du, L. B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater.2019, 84, 367–377.
Jiang, X. C.; Xiang, J. J.; Wu, H. H.; Zhang, T. Y.; Zhang, D. P.; Xu, Q. H.; Huang, X. L.; Kong, X. L.; Sun, J. H.; Hu, Y. L. et al. Neural stem cells transfected with reactive oxygen species-responsive polyplexes for effective treatment of ischemic stroke. Adv. Mater.2019, 31, 1807591.
Lu, Y. F.; Li, C.; Chen, Q. J.; Liu, P. X.; Guo, Q.; Zhang, Y.; Chen, X. L.; Zhang, Y. J.; Zhou, W. X.; Liang, D. H. et al. Microthrombustargeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv. Mater.2019, 31, 1808361.
Fan, Q.; Chen, M. L.; Fang, X. Y.; Lau, W. B.; Xue, L.; Zhao, L. N.; Zhang, H.; Liang, Y. H.; Bai, X.; Niu, H. Y. et al. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. AGE2013, 35, 1017–1026.
Moloney, J. N.; Cotter, T. G. ROS signalling in the biology of cancer. Semin. Cell Develop. Biol.2018, 80, 50–64.
El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and mutagenesis. Cancer Cell2017, 32, 727–729.
Manton, K. G.; Volovik, S.; Kulminski, A. ROS effects on neurodegeneration in Alzheimer’s disease and related disorders: On environmental stresses of ionizing radiation. Curr. Alzheimer Res.2004, 1, 277–293.
Lan, A. P.; Chen, J.; Chai, Z. F.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. BioMetals2016, 29, 665–678.
Li, P. Y.; Stetler, R. A.; Leak, R. K.; Shi, Y. J.; Li, Y.; Yu, W. F.; Bennett, M. V. L.; Chen, J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology2018, 134, 208–217.
Hatefi, Y. ATP synthesis in mitochondria. Eur. J. Biochem.1993, 218, 759–767.
Nicholls, D. G. Mitochondrial membrane potential and aging. Aging Cell2004, 3, 35–40.
Hüttemann, M.; Lee, I.; Pecinova, A.; Pecina, P.; Przyklenk, K.; Doan, J. W. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr.2008, 40, 445.
Susin, S. A.; Zamzami, N.; Castedo, M.; Daugas, E.; Wang, H. G.; Geley, S.; Fassy, F.; Reed, J. C.; Kroemer, G. The central executioner of apoptosis: Multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J. Exp. Med.1997, 186, 25–37.
Kubli, D. A.; Gustafsson, A. B. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res.2012, 111, 1208–1221.
Kolosowska, N.; Keuters, M. H.; Wojciechowski, S.; Keksa-Goldsteine, V.; Laine, M.; Malm, T.; Goldsteins, G.; Koistinaho, J.; Dhungana, H. Peripheral administration of IL-13 induces antiinflammatory microglial/macrophage responses and provides neuroprotection in ischemic stroke. Neurotherapeutics2019, 16, 1304–1319.
Subramaniam, S. R.; Chesselet, M. F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol.2013, 106-107, 17–32.
Hauser, D. N.; Hastings, T. G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis.2013, 51, 35–42.
Mondragón-Rodríguez, S.; Perry, G.; Zhu, X. W.; Moreira, P. I.; Acevedo-Aquino, M. C.; Williams, S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: Implications for Alzheimer’s disease. Oxid. Med. Cell. Longev.2013, 2013, 940603.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 31571020, 31570856 and 21375133), and Beijing Nova Programme Interdisciplinary Cooperation Project (No. Z191100001119002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflicts of interest. None of the authors has a financial conflict of interest related to this study.
Electronic Supplementary Material
12274_2020_2930_MOESM1_ESM.pdf
The enhanced protective effects of salvianic acid A: A functionalized nanoparticles against ischemic stroke through increasing the permeability of the blood-brain barrier
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, X., Qi, Z. et al. The enhanced protective effects of salvianic acid A: A functionalized nanoparticles against ischemic stroke through increasing the permeability of the blood-brain barrier. Nano Res. 13, 2791–2802 (2020). https://doi.org/10.1007/s12274-020-2930-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2930-6