Abstract
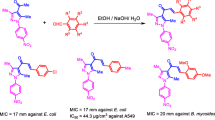
A new series of naphthyl chalcones (3a–3p) and their pyrazoline derivatives (4a–4h) were synthesized using substituted acetophenones, substituted naphthaldehydes, and hydrazine hydrate as starting materials. All the synthesized compounds were characterized by IR, NMR, and mass spectrometric analysis and screened for antimycobacterial activity against Mycobacterium tuberculosis H37Rv (ATCC 27924) and antibacterial activity against Staphylococcus aureus (MTCC 96), Bacillus subtilis (MTCC 441), Escherichia coli (MTCC 443) and Klebsiella pneumonia (MTCC 109). Compounds 3b and 3p exhibited significant antibacterial activity against all the tested bacterial strains. Amongst the synthesized compounds, compound 4b with 2-hydroxy-5-bromophenyl substitution at 3rd position of pyrazoline showed significant antimycobacterial activity with MIC of 6.25 µM comparable to that of standard isoniazid. The synthesized compounds were further screened for their cytotoxic activity against the MDA-MB-231 and SKOV3 cell lines. The compounds 3a, 3l, 4b, 4c, 4e, and 4h did not exhibit any cytotoxicity, and other compounds exhibited IC50 values higher than 8 and 22 µM against MDA-MB-231 and SKOV3 cell lines, respectively, compared to 1.20 and 1.30 µM shown by standard doxorubicin. To find out the putative binding mode of significantly active and weakly active compounds, a molecular docking study was also performed. In that, the most active compound 4b, displayed a hydrogen bond interaction with docking score of −10.50 kcal/mol and energy of −44.50 weakly active compound 3h did not show any crucial hydrogen bond interaction with the surrounded amino-acid residues and revealed a docking score of −6.74 and docking energy of −42.50.











Similar content being viewed by others
References
Ahmad A, Husain A (2016) Synthesis, antimicrobial, and antitubercular activities of some novel pyrazoline derivatives. J Saudi Chem Soc 20:577–584. https://doi.org/10.1016/j.jscs.2014.12.004
Ahmad I, Prakash J, Chanda D et al. (2013) Syntheses of lipophilic chalcones and their conformationally restricted analogs as antitubercular agents. Bioorg Med Chem Lett 23:1322–1325. https://doi.org/10.1016/j.bmcl.2012.12.096
Burmaoglu S, Algul O, Gobek et al. (2017) Design of potent fluoro-substituted chalcones as antimicrobial agents. J Enzym Inhib Med Chem 32:490–495. https://doi.org/10.1080/14756366.2016.1265517
Casey JM, GJ. Carlson, JW. Ford, TE. Strecker, E Hamel, Mary Lynn Trawick KGP (2019) Synthesis and biological evaluation of structurally diverse α-conformationally restricted chalcones and related analogues. MedChemComm 1–26. https://doi.org/10.1039/C9MD00127A.
Castaño LF, Cuartas V, Bernal A et al. (2019) New chalcone-sulfonamide hybrids exhibiting anticancer and antituberculosis activity. Eur J Med Chem 176:50–60. https://doi.org/10.1016/j.ejmech.2019.05.013
Chiaradia LD, Graziela P, Martins A et al. (2012) Synthesis, biological evaluation, and molecular modeling of chalcone derivatives as potent inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatases (PtpA and PtpB). J Med Chem 55:390–402
Farah SI, Abdelrahman AA, North EJ, Chauhan H (2015) Opportunities and challenges for natural products as novel antituberculosis agents. Mary Ann Liebert, Inc. XX:14–16. https://doi.org/10.1089/adt.2015.673.
Figueroa-valverde L, Hau-heredia L, García-cervera E et al. (2017) Activity exerted by a naphthalene-oxirane derivative on the ischemia/reperfusion injury. Biomed Res 28:16–22
Gomes MN, Braga RC, Grzelak EM et al. (2017) QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. Eur J Med Chem 137:126–138. https://doi.org/10.1016/j.ejmech.2017.05.026
Hans RH, Guantai EM, Lategan C et al. (2010) Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg Med Chem Lett 20:942–944. https://doi.org/10.1016/j.bmcl.2009.12.062
He X, Alian A, Stroud R, Montellano PRO De (2006) Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis. 6308–6323. https://doi.org/10.1021/jm060715y.
Khan SA, Asiri AM (2017) Green synthesis, characterization and biological evaluation of novel chalcones as anti bacterial agents. Arab J Chem 10:S2890–S2895. https://doi.org/10.1016/j.arabjc.2013.11.018
Kumar D, Ahmad I, Shukla A, Khan F (2014) QSAR and docking studies on chalcone derivatives for antitubercular activity against M. tuberculosis H 37 Rv. J Chemom 1–24. https://doi.org/10.1002/cem.2606.
Kumar RK, Sharma RSK, Kumar DR et al. (2016) ISSN 0975-413X CODEN (USA): PCHHAX characterization, synthesis and biological evaluation of naphthalene based piperazines as anti bacterial agents. Der Pharma Chem 8:374–379
Lin Y, Zhou Y, Flavin MT et al. (2002) Chalcones and flavonoids as anti-tuberculosis agents. Bioorg Med Chem 10:2795–2802
Lopes T, Ventura B, Calixto SD et al. (2015) Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20:8072–8093. https://doi.org/10.3390/molecules20058072
Malothu N, Bhandaru JS, Kulandaivelu U et al. (2015) Synthesis, in vitro antimycobacterial evaluation and docking studies of some new 5,6,7,8-tetrahydropyrido[4′,3′:4,5]thieno[2,3-d]pyrimidin-4(3H)-one Schiff Bases. Bioorg Med Chem Lett 4. https://doi.org/10.1016/j.bmcl.2015.12.083.
Marrapu VK, Chaturvedi V, Singh S et al. (2011) Novel aryloxy azolyl chalcones with potent activity against Mycobacterium tuberculosis H37Rv. Eur J Med Chem 46:4302–4310. https://doi.org/10.1016/j.ejmech.2011.06.037
Nayyar A, Malde A, Jain R (2006) Synthesis, anti-tuberculosis activity, and 3D-QSAR study of ring-substituted-2/4-quinolinecarbaldehyde derivatives. Bioorg Med Chem 14:7302–7310. https://doi.org/10.1016/j.bmc.2006.06.049
Patole J, Shingnapurkar D, Ratledge C (2006) Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Bioorg Med Chem Lett 16:1514–1517. https://doi.org/10.1016/j.bmcl.2005.12.035
Rachakonda V, Alla M, Sravanti S, Ummani R (2013) Design, diversity-oriented synthesis and structure activity relationship studies of quinolinyl heterocycles as antimycobacterial agents. Eur J Med Chem 70:536–547. https://doi.org/10.1016/j.ejmech.2013.10.034
Rokade S, Agrawal S, Shastri J (2010) Antimicrobial susceptibility testing of rapidly growing mycobacteria by microdilution—experience of a tertiary care centre. Indian J Med Microbiol 28:48–51. https://doi.org/10.4103/0255-0857.58729
Sharma M, Chaturvedi V, Manju YK et al. (2009) Substituted quinolinyl chalcones and quinolinyl pyrimidines as a new class of anti-infective agents. Eur J Med Chem 44:2081–2091. https://doi.org/10.1016/j.ejmech.2008.10.011
Sivakumar PM, Seenivasan SP, Doble M (2007) Synthesis, antimycobacterial activity evaluation, and QSAR studies of chalcone derivatives. Bioorg Med Chem Lett 17:1695–1700. https://doi.org/10.1016/j.bmcl.2006.12.112
Zhou B, He Y, Zhang X et al. (2010) Targeting mycobacterium protein tyrosine phosphatase B for antituberculosis agents. PNAS 107:4573–4578. https://doi.org/10.1073/pnas.0909133107
Acknowledgements
One of the authors thankful to the Central Mass Division, IICT, Hyderabad for providing mass spectrometry, Molecular Biology Department for providing the antimycobacterial activity. BKK and SM thankful to the Department of Biotechnology, Indo-Spain, New Delhi (Ref. No: BT/IN/Spain/39/SM/2017-2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pola, S., Banoth, K.K., Sankaranarayanan, M. et al. Design, synthesis, in silico studies, and evaluation of novel chalcones and their pyrazoline derivatives for antibacterial and antitubercular activities. Med Chem Res 29, 1819–1835 (2020). https://doi.org/10.1007/s00044-020-02602-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02602-8