Abstract
Purpose
Our purpose was to identify human ovarian extracellular matrix (ECM) components that would support in vitro culture of human ovarian tissue and be compatible with possible future clinical applications. We characterized ovarian expression of laminins and selected three laminin tripeptides for culture experiments to be compared with Matrigel, an undefined and animal-based mixture of ECM components.
Methods
Expression of the 12 laminin genes was determined on transcript and protein levels using cortical tissue samples (n = 6), commercial ovary RNA (n = 1), follicular fluid granulosa cells (n = 20), and single-cell RNA-sequencing data. Laminin 221 (LN221), LN521, LN511, and their mixture were chosen for a 7-day culture experiment along with Matrigel using tissue from 17 patients. At the end of the culture, follicles were evaluated by scoring and counting from serial tissue sections, apoptosis measured using in situ TUNEL assay, proliferation by Ki67 staining, and endocrine function by quantifying steroids in culture media using UPLC-MS/MS.
Results
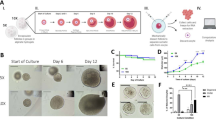
Approximately half of the cells in ovarian cortex expressed at least one laminin gene. The overall most expressed laminin α-chains were LAMA2 and LAMA5, β-chains LAMB1 and LAMB2, and γ-chain LAMC1. In culture experiments, LN221 enhanced follicular survival compared with Matrigel (p < 0.001), whereas tissue cultured on LN521 had higher proportion of secondary follicles (p < 0.001). LN511 and mixture of laminins did not support the cultures leading to lower follicle densities and higher apoptosis. All cultures produced steroids and contained proliferating cells.
Conclusions
LN221 and LN521 show promise in providing xeno-free growth substrates for human ovarian tissue cultures, which may help in further development of folliculogenesis in vitro for clinical practices. The system could also be used for identification of adverse effects of chemicals in ovaries.





Similar content being viewed by others
Availability of data and material
Data related to patient samples cannot be shared due to ethical and personal data protection restrictions. All materials (except human ovarian tissue) are commercially available.
References
Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59(4):783–90.
Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12(5):1032–6.
Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–8. https://doi.org/10.1093/humrep/den070.
McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24(3):135–42. https://doi.org/10.1093/molehr/gay002.
Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31(8):1013–28. https://doi.org/10.1007/s10815-014-0252-x.
Yin H, Kristensen SG, Jiang H, Rasmussen A, Andersen CY. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum Reprod. 2016;31(7):1531–9. https://doi.org/10.1093/humrep/dew049.
Pors SE, Ramlose M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, et al. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod. 2019;34(8):1523–35. https://doi.org/10.1093/humrep/dez077.
Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. https://doi.org/10.1038/ncomms15261.
Paulini F, Vilela JM, Chiti MC, Donnez J, Jadoul P, Dolmans MM, et al. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod BioMed Online. 2016;33(3):425–32. https://doi.org/10.1016/j.rbmo.2016.05.003.
Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. https://doi.org/10.1038/srep17323.
Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207.
Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. https://doi.org/10.1038/nature20104.
Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24(4):262–9. https://doi.org/10.1055/s-2006-948555.
Scott JE, Carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod BioMed Online. 2004;9(3):287–93.
Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84(4):689–97. https://doi.org/10.1095/biolreprod.110.088674.
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–86. https://doi.org/10.1016/j.semcancer.2005.05.004.
Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–53. https://doi.org/10.1146/annurev-cellbio-101011-155750.
Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. https://doi.org/10.1038/ncomms4195.
Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24(4):195–203. https://doi.org/10.1055/s-2006-948549.
Irving-Rodgers HF, Hummitzsch K, Murdiyarso LS, Bonner WM, Sado Y, Ninomiya Y, et al. Dynamics of extracellular matrix in ovarian follicles and corpora lutea of mice. Cell Tissue Res. 2010;339(3):613–24. https://doi.org/10.1007/s00441-009-0905-8.
Irving-Rodgers HF, Friden BE, Morris SE, Mason HD, Brannstrom M, Sekiguchi K, et al. Extracellular matrix of the human cyclic corpus luteum. Mol Hum Reprod. 2006;12(9):525–34. https://doi.org/10.1093/molehr/gal060.
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. https://doi.org/10.1186/1471-2105-13-134.
Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. https://doi.org/10.1038/s41467-020-14936-3.
Hao J, Tuck AR, Sjodin MOD, Lindberg J, Sand A, Niklasson B, et al. Resveratrol supports and alpha-naphthoflavone disrupts growth of human ovarian follicles in an in vitro tissue culture model. Toxicol Appl Pharmacol. 2018;338:73–82. https://doi.org/10.1016/j.taap.2017.11.009.
Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018.
team R. RStudio. Integrated Development for R. Boston: RStudio, Inc; 2019.
Ouni E, Vertommen D, Chiti MC, Dolmans MM, Amorim CA. A draft map of the human ovarian proteome for tissue engineering and clinical applications. Mol Cell Proteomics. 2019;18(Suppl 1):S159–S73. https://doi.org/10.1074/mcp.RA117.000469.
Baert Y, Stukenborg JB, Landreh M, De Kock J, Jornvall H, Soder O, et al. Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod. 2015;30(2):256–67. https://doi.org/10.1093/humrep/deu330.
Kanninen LK, Harjumaki R, Peltoniemi P, Bogacheva MS, Salmi T, Porola P, et al. Laminin-511 and laminin-521-based matrices for efficient hepatic specification of human pluripotent stem cells. Biomaterials. 2016;103:86–100. https://doi.org/10.1016/j.biomaterials.2016.06.054.
Tjin MS, Chua AWC, Moreno-Moral A, Chong LY, Tang PY, Harmston NP, et al. Biologically relevant laminin as chemically defined and fully human platform for human epidermal keratinocyte culture. Nat Commun. 2018;9(1):4432. https://doi.org/10.1038/s41467-018-06934-3.
Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat Protoc. 2018;13(7):1662–85. https://doi.org/10.1038/s41596-018-0007-8.
Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not −332, −111, or −411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26(11):2800–9. https://doi.org/10.1634/stemcells.2007-0389.
Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356(1–2):24–30. https://doi.org/10.1016/j.mce.2011.05.027.
Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. https://doi.org/10.1038/ncomms14584.
Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, et al. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet. 2018;35(1):41–8. https://doi.org/10.1007/s10815-017-1091-3.
Manavella DD, Cacciottola L, Pomme S, Desmet CM, Jordan BF, Donnez J, et al. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum Reprod. 2018;33(6):1107–16. https://doi.org/10.1093/humrep/dey080.
Acknowledgments
The authors would like to thank all patients who donated their ovarian tissue and follicular fluid, without which this study would not have been possible. We would also like to thank the midwives and clinicians who assisted with patient recruitment and logistics of tissue collection. We acknowledge Richelle Duque Björvang and Magdalena Wagner for their valuable input in writing of this manuscript. We thank Raoul Kuiper and Tarja Schröder from the Morphological Phenotype Analysis facility, Karolinska Institutet, Sweden, for H&E stains and tissue scans.
Funding
This study was funded by research grants from Swedish Research Council (#2017-02316), Swedish Research Council FORMAS (#2015-00623 and #2016-02031), and Swedish Childhood Cancer Foundation (#PR2017-0044).
Author information
Authors and Affiliations
Contributions
JH: preparation of samples, experimental design, data collection and analysis, and drafting of the manuscript; AT: data analysis and drafting of the manuscript; CH: TUNEL experiments and data analysis; AD: bioinformatic analyses; MODS and JL: steroid analysis; BN and KP: coordination of sample collection; OH: experimental design and interpretation of data; PD: preparation of samples, experimental design, data analysis, and writing of the manuscript. All authors participated in the writing of the manuscript and approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Stockholm Region Ethics Board (license numbers 2010/549-31/2 and 2015/798-31/12).
Consent to participate
Informed consent was obtained from all individuals included in the study.
Consent for publication
Not applicable.
Code availability
The code used to plot laminin expression in single-cell RNA-seq data is available upon request. The original RNA-seq data is described in Wagner et al. Nat Comm 2020 and has been deposited in the ArrayExpress database at EMBL-EBI under the accession code E-MTAb-8381.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, J., Tuck, A.R., Prakash, C.R. et al. Culture of human ovarian tissue in xeno-free conditions using laminin components of the human ovarian extracellular matrix. J Assist Reprod Genet 37, 2137–2150 (2020). https://doi.org/10.1007/s10815-020-01886-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01886-4