Abstract
Objective and design
To clarify the effects of dietary supplementation of protocatechuic acid (PCA) and in-depth mechanisms on allergic asthma in ovalbumin (OVA)-induced mice.
Materials
Female BALB/c mice were randomly divided into three groups (n = 10 in each group): control group, OVA-induced allergic asthma group, and OVA plus PCA group.
Treatment
Dietary supplementation of PCA was achieved by adding 50 mg/kg PCA to AIN 93G diet for 25 days.
Methods
Peripheral blood cells, pulmonary inflammatory cell infiltration, the levels of IL-4, IL-5, and IL-13 in bronchoalveolar lavage fluid (BALF), the mRNA levels of Th2-related genes in the lungs, and the protein expressions of the IL-4Rα–STAT6 and the Jagged1/Jagged2–Notch1/Notch2 signaling pathways were measured.
Results
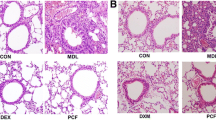
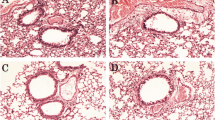
Significantly reduced inflammatory cells infiltration and mucosal hypersecretion in the lung tissues, repaired levels of interleukin IL-4, IL-5, and IL-13 in the BALF, and decreased mRNA expression of IL-4, IL-5, and GATA3 were observed in OVA plus PCA group. Moreover, PCA treatment down-regulated the protein levels of IL-4Rα–STAT6 and Jagged1/Jagged2–Notch1/Notch2 signaling pathways.
Conclusions
Dietary supplement of PCA alleviated allergic asthma partly through suppressing the IL-4Rα–STAT6 and Jagged1/Jagged2–Notch1/Notch2 signaling pathways in mice. Our study provided the theoretic basis of PCA used as functional food in preventing allergic asthma.






Similar content being viewed by others
References
Loftus PA, Wise SK. Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24(3):245–9.
Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35–50.
Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):116–24.
Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65.
Galván CA, Guarderas JC. Practical considerations for dysphonia caused by inhaled corticosteroids. Mayo Clin Proc. 2012;87(9):901–4.
Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367(10):904–12.
Turpeinen M, Pelkonen AS, Nikander K, Sorva R, Selroos O, Juntunen-Backman K, et al. Bone mineral density in children treated with daily or periodical inhaled budesonide: the helsinki early intervention childhood asthma study. Pediatr Res. 2010;68(2):169–73.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Nair P. Anti-interleukin-5 monoclonal antibody to treat severe eosinophilic asthma. N Engl J Med. 2014;371(13):1249–51.
Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170(6):583–93.
Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP, Group IPTS. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) phase two. Thorax. 2010;65(6):516–22.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73.
Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114(2):270–9.
Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, et al. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014;69(10):1380–9.
Yokota-Nakatsuma A, Takeuchi H, Ohoka Y, Kato C, Song SY, Hoshino T, et al. Retinoic acid prevents mesenteric lymph node dendritic cells from inducing IL-13-producing inflammatory Th2 cells. Mucosal Immunol. 2014;7(4):786–801.
Aswar UM, Kandhare AD, Mohan V, Thakurdesai PA. Anti-allergic effect of intranasal administration of type-A procyanidin polyphenols based standardized extract of cinnamon bark in ovalbumin sensitized BALB/c mice. Phytother Res. 2015;29(3):423–33.
Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Gammazza AM, Knap N, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19(3):686.
Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016;21(5):623.
Simpson T, Kure C, Stough C. Assessing the efficacy and mechanisms of pycnogenol® on cognitive aging from animal and human studies. Front Pharmacol. 2019;10:694.
Belcaro G, Luzzi R, Cesinaro Di Rocco P, Cesarone MR, Dugall M, Feragalli B, et al. Pycnogenol® improvements in asthma management. Panminerva Med. 2011;53(3 Suppl 1):57–64.
Kay CD, Pereira-Caro G, Ludwig IA, Clifford MN, Crozier A. Anthocyanins and flavanones are more bioavailable than previously perceived: a review of recent evidence. Annu Rev Food Sci Technol. 2017;8:155–80.
Chen BL, Chen YQ, Ma BH, Yu SF, Li LY, Zeng QX, et al. Tetrahydrocurcumin, a major metabolite of curcumin, ameliorates allergic airway inflammation by attenuating Th2 response and suppressing the IL-4Ralpha-Jak1-STAT6 and Jagged1/Jagged2 -Notch1/Notch2 pathways in asthmatic mice. Clin Exp Allergy. 2018;48(11):1494–508.
Ma BH, Wu YF, Chen BL, Yao YL, Wang YY, Bai HL, et al. Cyanidin-3-O-beta-glucoside attenuates allergic airway inflammation by modulating the IL-4R alpha-STAT6 signaling pathway in a murine asthma model. Int Immunopharmacol. 2019;69:1–10.
Pyo MY, Yoon SJ, Yu Y, Park S, Jin M. Cyanidin-3-glucoside suppresses Th2 cytokines and GATA-3 transcription factor in EL-4 T cells. Biosci Biotechnol Biochem. 2014;78(6):1037–43.
Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137(9):2043–8.
Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943.
Wei M, Chu X, Jiang L, Yang X, Cai Q, Zheng C, et al. Protocatechuic acid attenuates lipolysaccharide-induced acute lung injury. Inflammation. 2012;35(3):1169–78.
Wei M, Chu X, Guan M, Yang X, Xie X, Liu F, et al. Protocatechuic acid suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Int Immunopharmacol. 2013;15(4):780–8.
Bogaert P, Naessens T, De Koker S, Hennuy B, Hacha J, Smet M, et al. Inflammatory signatures for eosinophilic vs. neutrophilic allergic pulmonary inflammation reveal critical regulatory checkpoints. Am J Physiol Lung Cell Mol Physiol. 2011;300(5):L679–90.
Huang WC, Fang LW, Liou CJ. Phloretin attenuates allergic airway inflammation and oxidative stress in asthmatic mice. Front Immunol. 2017;8:134.
Zhang XY, Simpson JL, Powell H, Yang IA, Upham JW, Reynolds PN, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy. 2014;44(9):1137–45.
Huang WC, Chan CC, Wu SJ, Chen LC, Shen JJ, Kuo ML, et al. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J Ethnopharmacol. 2014;151(1):470–7.
Fish SC, Donaldson DD, Goldman SJ, Williams CM, Kasaian MT. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J Immunol. 2005;174(12):7716–24.
Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–61.
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386(9998):1086–96.
Takeda K, Kishimoto T, Akira S. STAT6: its role in interleukin 4-mediated biological functions. J Mol Med. 1997;75(5):317–26.
Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380(6575):627–30.
Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380(6575):630–3.
Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–96.
Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38.
Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10(10):493–9.
Foster PS, Webb DC, Yang M, Herbert C, Kumar RK. Dissociation of T helper type 2 cytokine-dependent airway lesions from signal transducer and activator of transcription 6 signalling in experimental chronic asthma. Clin Exp Allergy. 2003;33(5):688–95.
Blease K, Schuh JM, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, et al. Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol. 2002;160(2):481–90.
Tindemans I, Lukkes M, de Bruijn MJW, Li BWS, van Nimwegen M, Amsen D, et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J Allergy Clin Immunol. 2017;140(4):1079–89.
Li-Weber M, Krammer PH. Regulation of IL4 gene expression by T cells and therapeutic perspectives. Nat Rev Immunol. 2003;3(7):534–43.
Li-Weber M, Salgame P, Hu C, Davydov IV, Laur O, Klevenz S, et al. Th2-specific protein/DNA interactions at the proximal nuclear factor-AT site contribute to the functional activity of the human IL-4 promoter. J Immunol. 1998;161(3):1380–9.
Rooney JW, Hoey T, Glimcher LH. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity. 1995;2(5):473–83.
Wu Y, Wu T, Xu B, Xu X, Chen H, Li X, et al. Protocatechuic acid inhibits osteoclast differentiation and stimulates apoptosis in mature osteoclasts. Biomed Pharmacother. 2016;82:399–405.
Masella R, Santangelo C, D'Archivio M, Li Volti G, Giovannini C, Galvano F. Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr Med Chem. 2012;19(18):2901–17.
Espin JC, González-Sarrías A, Tomás-Barberán FA. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93.
Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111(8):967–81.
Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010;10(8):959–66.
Wang D, Zou T, Yang Y, Yan X, Ling W. Cyanidin-3-O-beta-glucoside with the aid of its metabolite protocatechuic acid, reduces monocyte infiltration in apolipoprotein E-deficient mice. Biochem Pharmacol. 2011;82(7):713–9.
Yan JJ, Jung JS, Hong YJ, Moon YS, Suh HW, Kim YH, et al. Protective effect of protocatechuic acid isopropyl ester against murine models of sepsis: inhibition of TNF-alpha and nitric oxide production and augmentation of IL-10. Biol Pharm Bull. 2004;27(12):2024–7.
McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51(6):702–13.
Scott HA, Jensen ME, Wood LG. Dietary interventions in asthma. Curr Pharm Des. 2014;20(6):1003–100.
Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96(3):534–43.
Gref A, Rautiainen S, Gruzieva O, Håkansson N, Kull I, Pershagen G, et al. Dietary total antioxidant capacity in early school age and subsequent allergic disease. Clin Exp Allergy. 2017;47(6):751–9.
Patel BD, Welch AA, Bingham SA, Luben RN, Day NE, Khaw KT, et al. Dietary antioxidants and asthma in adults. Thorax. 2006;61(5):388–93.
Patel S, Murray CS, Woodcock A, Simpson A, Custovic A. Dietary antioxidant intake, allergic sensitization and allergic diseases in young children. Allergy. 2009;64(12):1766–72.
Soutar A, Seaton A, Brown K. Bronchial reactivity and dietary antioxidants. Thorax. 1997;52(2):166–70.
Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356(9241):1573–4.
Acknowledgements
Qin Li and Yinfan Wu have contributed equally to this work.
Funding
This study was supported by Guangzhou Science and Technology Program [201804020045], the Laboratory Open Found of Sun Yat-sen University [20180278], Shenzhen Science and Technology Innovation Commission [201803073000433], and National Natural Science Foundation of China [81573145 and 81730090].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Q., Wu, Y., Guo, X. et al. Protocatechuic acid supplement alleviates allergic airway inflammation by inhibiting the IL-4Rα–STAT6 and Jagged 1/Jagged2–Notch1/Notch2 pathways in allergic asthmatic mice. Inflamm. Res. 69, 1027–1037 (2020). https://doi.org/10.1007/s00011-020-01379-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01379-1