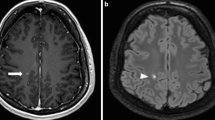
Abstract
Pediatric-onset multiple sclerosis (POMS) is defined by a first multiple sclerosis (MS) attack occurring before 18 years old and is diagnosed by demonstration of dissemination in time (DIT) and space (DIS). Although guidelines evolved over the years, they always recognized the importance of magnetic resonance imaging (MRI) for diagnosis. The 2017 McDonald criteria are increasingly used and have been validated in several cohorts. The use of MRI is the most important tool for the early diagnosis, monitoring, and assessment of treatment response of MS and standard protocols include precontrast and postcontrast T1, T2, fluid attenuation inversion recovery (FLAIR) and diffusion sequences. A distinctive MS lesion compromises white matter and it is well-demarcated and confluent, showing demyelination, inflammation, gliosis, and relative axonal preservation. Considering the growing recognition of pediatric MS as a differential diagnosis for children presenting with a clinical central nervous system event, we present a POMS lesions guide (periventricular, juxtacortical, infratentorial, spinal cord, cortical, tumefactive, black hole, contrast-enhanced). Owing to its rareness, POMS is a diagnosis by exclusion and MRI plays a fundamental role in distinguishing POMS from other demyelinating and non-demyelinating conditions. Three main groups of disorders can mimic POMS: inflammatory, metabolic and tumoral; however, imaging patterns earlier described lower the possibilities of alternative diagnoses and strongly suggest POMS when likely.








Similar content being viewed by others
References
Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008;7:841–51.
Tenembaum SN. Pediatric Multiple Sclerosis: Distinguishing Clinical and MR Imaging Features. Neuroimaging Clin N Am. 2017;27:229–50.
Jeong A, Oleske DM, Holman J. Epidemiology of Pediatric-Onset Multiple Sclerosis: A Systematic Review of the Literature. J Child Neurol. 2019;34:705–12.
Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389:1336–46.
Chabas D, Castillo-Trivino T, Mowry EM, Strober J, Glenn O, Waubant E. Vanishing MS T2-bright lesions before puberty: a distinct MRI phenotype? Neurology. 2008;71:1090–3.
Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E; International Pediatric Multiple Sclerosis Study Group. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–7.
Peche SS, Alshekhlee A, Kelly J, Lenox J, Mar S. A long-term follow-up study using IPMSSG criteria in children with CNS demyelination. Pediatr Neurol. 2013;49:329–34.
Mikaeloff Y, Adamsbaum C, Husson B, Vallée L, Ponsot G, Confavreux C, et al. MRI prognostic factors for relapse after acute CNS inflammatory demyelination in childhood. Brain. 2004;127:1942–7.
Callen DJ, Shroff MM, Branson HM, Lotze T, Li DK, Stephens D, Banwell BL. MRI in the diagnosis of pediatric multiple sclerosis. Neurology. 2009;72:961–7.
Verhey LH, Branson HM, Shroff MM, Callen DJ, Sled JG, Narayanan S, Sadovnick AD, Bar-Or A, Arnold DL, Marrie RA, Banwell B; Canadian Pediatric Demyelinating Disease Network. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol. 2011;10:1065–73.
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73.
Wong YYM, de Mol CL, van der Vuurst de Vries RM, van Pelt ED, Ketelslegers IA, Catsman-Berrevoets CE, Neuteboom RF, Hintzen RQ. Real-world validation of the 2017 McDonald criteria for pediatric MS. Neurol Neuroimmunol Neuroinflamm. 2018;6:e528.
Hacohen Y, Brownlee W, Mankad K, Chong WK’, Thompson A, Lim M, Wassmer E, Hemingway C, Barkhof F, Ciccarelli O. Improved performance of the 2017 McDonald criteria for diagnosis of multiple sclerosis in children in a real-life cohort. Mult Scler. 2019. https://doi.org/10.1177/1352458519863781. Epub ahead of print.
McKay KA, Hillert J, Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology. 2019;92:e2764–73.
Waldman A, Ness J, Pohl D, Simone IL, Anlar B, Amato MP, Ghezzi A. Pediatric multiple sclerosis: Clinical features and outcome. Neurology. 2016;87(9 Suppl 2):S74–81.
Kaunzner UW, Gauthier SA. MRI in the assessment and monitoring of multiple sclerosis: an update on best practice. Ther Adv Neurol Disord. 2017;10:247–61.
Wattjes MP, Barkhof F. High field MRI in the diagnosis of multiple sclerosis: high field–high yield? Neuroradiology. 2009;51:279–92.
Banwell B, Arnold DL, Tillema JM, Rocca MA, Filippi M, Weinstock-Guttman B, Zivadinov R, Sormani MP. MRI in the evaluation of pediatric multiple sclerosis. Neurology. 2016;87(9 Suppl 2):S88–96.
Barkovich AJ, Raybaud C. Pediatric neuroimaging. Philadelphia: Lippincott Williams & Wilkins; 2018.
Enzinger C, Barkhof F, Ciccarelli O, Filippi M, Kappos L, Rocca MA, Ropele S, Rovira À, Schneider T, de Stefano N, Vrenken H, Wheeler-Kingshott C, Wuerfel J, Fazekas F; MAGNIMS study group. Nonconventional MRI and microstructural cerebral changes in multiple sclerosis. Nat Rev Neurol. 2015;11:676–86.
Suthiphosuwan S, Sati P, Guenette M, Montalban X, Reich DS, Bharatha A, Oh J. The Central Vein Sign in Radiologically Isolated Syndrome. Version 2. AJNR Am J Neuroradiol. 2019;40:776–83.
Uysal E, Erturk SM, Yildirim H, Seleker F, Basak M. Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol. 2007;188:697–702.
Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg D. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161:721–5.
Rovira À, Wattjes MP, Tintoré M, Tur C, Yousry TA, Sormani MP, De Stefano N, Filippi M, Auger C, Rocca MA, Barkhof F, Fazekas F, Kappos L, Polman C, Miller D, Montalban X; MAGNIMS study group. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11:471–82.
Fazekas F, Barkhof F, Filippi M, Grossman RI, Li DK, McDonald WI, McFarland HF, Paty DW, Simon JH, Wolinsky JS, Miller DH. The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology. 1999;53:448–56.
Bar-Or A, Hintzen RQ, Dale RC, Rostasy K, Brück W, Chitnis T. Immunopathophysiology of pediatric CNS inflammatory demyelinating diseases. Neurology. 2016;87:12–S9.
Bergsland N, Horakova D, Dwyer MG, Uher T, Vaneckova M, Tyblova M, Seidl Z, Krasensky J, Havrdova E, Zivadinov R. Gray matter atrophy patterns in multiple sclerosis: A 10-year source-based morphometry study. Neuroimage Clin. 2017;17:444–51.
Aliaga ES, Barkhof F. MRI mimics of multiple sclerosis. Handbook of clinical neurology, Vol. 122. Amsterdam: Elsevier; 2014. pp. 291–316.
Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, Rovira A, Sastre-Garriga J, Tintorè M, Frederiksen JL, Gasperini C, Palace J, Reich DS, Banwell B, Montalban X, Barkhof F; MAGNIMS Study Group. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15:292–303.
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Adèr HJ, Losseff N, Valk J. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120:2059–69.
Miki Y, Grossman RI, Udupa JK, Wei L, Kolson DL, Mannon LJ, Grossman M. Isolated U-fiber involvement in MS: preliminary observations. Neurology. 1998;50:1301–6.
Nelson F, Poonawalla A, Hou P, Huang F, Wolinsky J, Narayana P. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol. 2007;28:1645–9.
Filippi M, Rocca MA, Calabrese M, Sormani MP, Rinaldi F, Perini P, Comi G, Gallo P. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75:1988–94.
Sethi V, Yousry TA, Muhlert N, Ron M, Golay X, Wheeler-Kingshott C, Miller DH, Chard DT. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry. 2012;83:877–82.
Filippi M, Preziosa P, Meani A, Ciccarelli O, Mesaros S, Rovira A, et al. Prediction of Filippi M, Preziosa P, Meani A, Ciccarelli O, Mesaros S, Rovira A, Frederiksen J, Enzinger C, Barkhof F, Gasperini C, Brownlee W, Drulovic J, Montalban X, Cramer SP, Pichler A, Hagens M, Ruggieri S, Martinelli V, Miszkiel K, Tintorè M, Comi G, Dekker I, Uitdehaag B, Dujmovic-Basuroski I, Rocca MA. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol. 2018;17:133–42.
Gawne-Cain ML, O’Riordan JI, Thompson AJ, Moseley IF, Miller DH. Multiple sclerosis lesion detection in the brain: a comparison of fast fluid-attenuated inversion recovery and conventional T2-weighted dual spin echo. Neurology. 1997;49:364–70.
Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66:54–9.
Simon JH, Li D, Traboulsee A, Coyle PK, Arnold DL, Barkhof F, Frank JA, Grossman R, Paty DW, Radue EW, Wolinsky JS. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27:455–61.
Hua L, Donlon S, Sobhanian M, Portner S, Okuda D. Thoracic spinal cord lesions are influenced by the degree of cervical spine involvement in multiple sclerosis. Spinal Cord. 2015;53:520–5.
Riva D, Chiapparini L, Pollo B, Balestrini MR, Massimino M, Milani N. A case of pediatric tumefactive demyelinating lesion misdiagnosed and treated as glioblastoma. J Child Neurol. 2008;23:944–7.
Masdeu J, Quinto C, Olivera C, Tenner M, Leslie D, Visintainer P. Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology. 2000;54:1427–33.
Patriarca L, Torlone S, Ferrari F, Di Carmine C, Totaro R, di Cesare E, Splendiani A. Is size an essential criterion to define tumefactive plaque? MR features and clinical correlation in multiple sclerosis. Neuroradiol J. 2016;29:384–9.
van Walderveen MA, Kamphorst W, Scheltens P, van Waesberghe JH, Ravid R, Valk J, Polman CH, Barkhof F. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. 1998;50:1282–8.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG; International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89.
Deisenhammer F, Fitzner B, Zetterberg H, Zettl UK. The cerebrospinal fluid in multiple sclerosis. Front Immunol. 2019;10:726.
Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53:1107–14.
Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, Kleiter I, Chitnis T; GJCF International Clinical Consortium & Biorepository for Neuromyelitis Optica. Demographic and clinical features of neuromyelitis optica: A review. Mult Scler. 2015;21:845–53.
Tenembaum S, Chitnis T, Nakashima I, Collongues N, McKeon A, Levy M, Rostasy K. Neuromyelitis optica spectrum disorders in children and adolescents. Neurology. 2016;87(9 Suppl 2):S59–66.
Absoud M, Lim MJ, Appleton R, Jacob A, Kitley J, Leite MI, Pike MG, Vincent A, Wassmer E, Waters P, Woodhall M, Hemingway C, Palace J. Paediatric neuromyelitis optica: clinical, MRI of the brain and prognostic features. J Neurol Neurosurg Psychiatry. 2015;86:470–2.
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15.
Collongues N, Marignier R, Zéphir H, Papeix C, Fontaine B, Blanc F, Rodriguez D, Fleury M, Vukusic S, Pelletier J, Audoin B, Thouvenot E, Camu W, Barroso B, Ruet A, Brochet B, Vermersch P, Confavreux C, de Seze J. Long-term follow-up of neuromyelitis optica with a pediatric onset. Neurology. 2010;75:1084–8.
Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, Paul F, Jarius S, Aktas O, Elsone L, Mutch K, Levy M, Takai Y, Collongues N, Banwell B, Fujihara K, de Seze J. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. 2014;20:843–7.
Peña JA, Ravelo ME, Cruz M‑L, Montiel-Nava C. NMO in pediatric patients: brain involvement and clinical expression. Arq Neuropsiquiatr. 2011;69:34–8.
McKeon A, Lennon VA, Lotze T, Tenenbaum S, Ness JM, Rensel M, Kuntz NL, Fryer JP, Homburger H, Hunter J, Weinshenker BG, Krecke K, Lucchinetti CF, Pittock SJ. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71:93–100.
Cobo-Calvo Á, Ruiz A, D’Indy H, Poulat AL, Carneiro M, Philippe N, Durand-Dubief F, Deiva K, Vukusic S, Desportes V, Marignier R. MOG antibody-related disorders: common features and uncommon presentations. J Neurol. 2017;264:1945–55.
Baumann M, Grams A, Djurdjevic T, Wendel EM, Lechner C, Behring B, Blaschek A, Diepold K, Eisenkölbl A, Fluss J, Karenfort M, Koch J, Konuşkan B, Leiz S, Merkenschlager A, Pohl D, Schimmel M, Thiels C, Kornek B, Schanda K, Reindl M, Rostásy K. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. J Neurol. 2018;265:845–55.
Hacohen Y, Mankad K, Chong WK, Barkhof F, Vincent A, Lim M, Wassmer E, Ciccarelli O, Hemingway C. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology. 2017;89:269–78.
Baumann M, Sahin K, Lechner C, Hennes EM, Schanda K, Mader S, Karenfort M, Selch C, Häusler M, Eisenkölbl A, Salandin M, Gruber-Sedlmayr U, Blaschek A, Kraus V, Leiz S, Finsterwalder J, Gotwald T, Kuchukhidze G, Berger T, Reindl M, Rostásy K. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86:265–72.
Sepúlveda M, Armangue T, Martinez-Hernandez E, Arrambide G, Sola-Valls N, Sabater L, Téllez N, Midaglia L, Ariño H, Peschl P, Reindl M, Rovira A, Montalban X, Blanco Y, Dalmau J, Graus F, Saiz A. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016;263:1349–60.
Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, Lee KW, Waters P. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015;2:e163.
Metreau-Vastel J, Mikaeloff Y, Tardieu M, Koné-Paut I, Tran TA. Neurological involvement in paediatric Behçet’s disease. Neuropediatrics. 2010;41:228–34.
Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, Poll-The BT. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. 2012;7:51.
Deiva K, Mahlaoui N, Beaudonnet F, de Saint Basile G, Caridade G, Moshous D, Mikaeloff Y, Blanche S, Fischer A, Tardieu M. CNS involvement at the onset of primary hemophagocytic lymphohistiocytosis. Neurology. 2012;78:1150–6.
Brennan KC, Lowe LH, Yeaney GA. Pediatric central nervous system posttransplant lymphoproliferative disorder. AJNR Am J Neuroradiol. 2005;26:1695–7.
Deiva K. Pediatric onset multiple sclerosis. Rev Neurol (Paris). 2020;176:30–36.
Author information
Authors and Affiliations
Contributions
Catherine Adamsbaum had the idea for the article. Gonzalo Barraza performed the literature search, data analysis, and drafted the work. Kumaran Deiva, Béatrice Husson and Catherine Adamsbaum critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
G. Barraza, K. Deiva, B. Husson and C. Adamsbaum declare that they have no competing interests.
Ethical standards
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Barraza, G., Deiva, K., Husson, B. et al. Imaging in Pediatric Multiple Sclerosis. Clin Neuroradiol 31, 61–71 (2021). https://doi.org/10.1007/s00062-020-00929-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-020-00929-8