Abstract
Most of the plant viral diseases spread through vectors. In case of the persistently transmitted disease, there is a latent time of infection inside the vector after acquisition of the virus from the infected plant. Again, the plant after getting infectious agent shows an incubation time after the interaction with an infected vector before it becomes diseased. The goal of this work is to study the effect of both incubation delay and latent time on the dynamics of plant disease, and accordingly a delayed model has been proposed. The existence of the equilibria, basic reproductive number (\(\mathcal {R}_0\)) and stability of equilibria have been studied. This study shows the relevance of the presence of two time delays, which may lead to system stabilization.






Similar content being viewed by others
References
Al Basir F, Blyuss KB, Ray S (2018) Modelling the effects of awareness-based interventions to control the mosaic disease of Jatropha curcas. Ecol Complex 36:92–100
Anguelov R, Lubuma J, Dumont Y (2012) Mathematical analysis of vector-borne diseases on plants. In: 2012 IEEE 4th international symposium on plant growth modeling, simulation, visualization and applications, pp 22–29
Bailey NTJ (1975) The mathematical theory of infectious diseases and its applications, 2nd edn. Charles Griffin & Company Limited, London
Banerjee M, Takeuchi Y (2017) Maturation delay for the predators can enhance stable coexistence for a class of prey-predator models. J Theor Biol 412:154–71
Bruhn JA, Fry WE (1981) Analysis of Potato Late Blight epidemiology by simulation modelling. Psychopathology 71(6):612–616
Buonomo B, Cerasuolo M (2014) Stability and bifurcation in plant-pathogens interactions. Appl Math Comput 232:858–871
Cao J, Xiao M (2007) Stability and Hopf bifurcation in a simplified BAM neural network with two time delays. IEEE Trans Neural Netw 18(2):416–430
Chan M, Jeger MJ (1994) Analytical model of plant virus disease dynamics with roguing and replanting. J Appl Ecol 31:413–427
Cook DWM, Long PJ, Ganesh S (1999) The combined effect of delayed application of yeast biocontrol agents and fruit curing for the inhibition of the postharvest pathogen Botrytis cinerea in kiwifruit. Postharvest Biol Technol 16:233–243
Cooke KL (1979) Stability analysis for a vector disease model. Rocky Mountain J Math 9(1):31–42
Dietzgen RG, Mann KS, Johnson KN (2016) Plant virus-insect vector interactions: current and potential future research directions. Viruses 8(11):303
Duffus JE (1987) Whitefly transmission of plant viruses. In: Harris KF (ed) Current topics in vector research. Springer, New York, pp 73–91
Haber S, Maxwell DP, Gilbertson RL (1991) Bean golden mosaic. In: Hall R (ed) Compendium of bean diseases. American Phytopathological Society, Eagan, pp 42–43
Hassard BD, Hassard DB, Kazarinoff ND, Wan YH, Wan YW (1981) Theory and applications of Hopf bifurcation, vol 41. CUP Archive, Cambridge
Heffernan JM, Smith RJ, Wahl LM (2005) Perspectives on the basic reproductive ratio. J R Soc Interface 2(4):281–93
Henneberry TJ, Castle SJ (2001) Bemisia pest status, economics, biology and population dynamics. In: Harris KF, Smith OP, Duffus JE (eds) Virus insects plant interactions. Academic Press, San Diego, pp 247–278
Hohn T (2007) Plant virus transmission from the insect point of view. Proc Natl Acad Sci 104(46):17905–17906
Holt J, Jeger MJ, Thresh JM, Otim-Nape GW (1997) An epidemilogical model incorporating vector population dynamics applied to African cassava mosaic virus disease. J Appl Ecol 34(3):793–806
Horowitz AR (1986) Population dynamics of Bemisia tabaci (Gennadius): with special emphasis on cotton fields. Agric Ecosyst Environ 17:37–47
Jackson M, Chen B (2016) Modeling plant virus propagation with delays. J Comput Appl Math 309:611–621
Jiao J, Chen L (2008) Global attractivity of a stage-structure variable coefficients predator-prey system with time delay and impulsive perturbations on predators. Int J Biomath 1(2):197–208
Kern H (1956) Problems of incubation in plant diseases. Ann Rev Microbiol 10(1):351–368
Kuznetsov YA (2013) Elements of applied bifurcation theory, vol 112. Springer, Berlin
Legg JP (2008) African cassava mosaic disease. In: Mahy BWJ, van Regenmortel MHV (eds) Encyclopedia of virology, 3rd edn. Elsevier, Amsterdam, pp 30–36
Li Q, Dai Y, Guo X, Zhang X (2018) Hopf bifurcation analysis for a model of plant virus propagation with two delays. Adv Differ Equ 2018:259
Liu D, Raghothama KG, Hasegawa PM, Bressan RA (2012) Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci U S A 91:1888–1892
Madden LV, Hughes G, Van Den Bosch F (2007) The study of plant disease epidemics. APS Press, St Paul
Meng X, Li Z (2010) The dynamics of plant disease models with continuous and impulsive cultural control strategies. J Theor Biol 266(1):29–40
Moore SM, Borer ET, Hosseini PR (2009) Predators indirectly control vector-borne disease: linking predator-prey and host-pathogen models. J R Soc Interface 7(42):161–176
Murphy AM, Holcombe LJ, Carr JP (2000) Characteristics of salicylic acid-induced delay in disease caused by a necrotrophic fungal pathogen in tobacco. Physiol Mol Plant Pathol 57:47–54
Niehaus K, Kapp D, Pühler A (1993) Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti mutant. Planta 190:415–425
Okamoto KW, Amarasekare P (2012) The biological control of disease vectors. J Theor Biol 309:47–57
Pal PJ, Saha T, Sen M, Banerjee M (2012) A delayed predatorprey model with strong Allee effect in prey population growth. Nonlinear Dyn 68:23–42
Perko L (1996) Differential equations and dynamical systems. Springer, Berlin
Rakshit N, Basir FA, Banerjee A, Ray S (2019) Dynamics of plant mosaic disease propagation and the usefulness of roguing as an alternative biological control. Ecol Complex 38:15–23
Rimbaud L, Dallot S, Delaunay A, Borron S, Soubeyrand S, Thébaud G, Jacquot E (2015) Assessing the mismatch between incubation and latent periods for vector-borne diseases: the case of Sharka. Phytopathology 105(11):1408–1416
Ruan S (2001) Absolute stability, conditional stability and bifurcation in Kolmogorov-type predator-prey systems with discrete delays. Q Appl Math 59(1):159–73
Shi R, Zhao H, Tang S (2014) Global dynamic analysis of a vector-borne plant disease model. Adv Differ Equ 2014:59
Shun-xiang RE, Zhen-zhong WA, Bao-li QI, Yuan XI (2001) The pest status of Bemisia tabaci in China and non-chemical control strategies. Insect Sci 8(3):279–288
Siegel A, Ginoza W, Wildman SG (1957) The early events of infection with tobacco mosaic virus nucleic acid. Virology 3:554–559
Smrž J (1972) The effect of the transmission of the beet mosaic virus on the variability of its incubation period. Biol Plant 14:1–10
Suffert F, Thompson RN (2018) Some empirical arguments demonstrating that the latent period varies over the course of a plant disease epidemic. bioRxiv 148619
Van der Plank JE (1963) Plant diseases: epidemics and control. Academic Press, London
Venturino E, Roy PK, Basir FA, Datta A (2016) A model for the control of the mosaic virus disease in Jatropha curcas plantations. Energy Ecol Environ 1:360–369
Villa F (1992) New computer architectures as tools for ecological thought. Trends Ecol Evol 7(6):179–183
Yoshimura A, Kawasaki K, Takasu F, Togashi K, Futai K, Shigesada N (1999) Modeling the spread of pine wilt disease caused by nematodes with pine sawyers as vector. Ecology 80(5):1691–1702
Zhang T, Meng X, Song Y, Li Z (2012) Dynamical analysis of delayed plant disease models with continuous or impulsive cultural control strategies. Abstr Appl Anal. https://doi.org/10.1155/2012/428453
Acknowledgements
The authors wishes to thank the anonymous reviewer for his/her careful reading of the manuscript. Fahad Al Basir acknowledges the University Grants Commission, Govt. of India, for Dr. D S Kothari Postdoctoral fellowship, F No: MA/16-17/0029. Sagar Adhurya is thankful to the University Grants Commission, New Delhi, for funding his research by NET-JRF fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ezio Venturino: Member of the INdAM research group GNCS.
Appendices
Appendix A
We follow the method established in the paper by Heffernan et al. (2005) for calculating \(\mathcal {R}_0\).
We consider the next-generation matrix G which comprises two parts, namely F and V, where
where \({F}_i\) are the new infections, while the \({V}_i\) transfers of infections from one compartment to another. \(E_0\) is the disease-free equilibrium. We get \(\mathcal {R}_0=\frac{\Pi k\lambda \beta }{md^2}\), and it is the dominant eigenvalue of the matrix \({G} = {F}{V}^{-1}\).
Appendix B
We rewrite the model (1), without delay, as follows:
and consider \(\lambda \) as bifurcation parameter to check the change in stability of the disease-free equilibrium point \(E_2(K,0,\frac{\Pi }{d},0)\). Jacobian matrix for (22) evaluated at \(E_2\) is given by
This matrix has a zero eigenvalue when \(\lambda \,\equiv \,\lambda _*\,=\,\frac{d^2m}{K\Pi \beta }\). The system undergoes a transcritical bifurcation as \(\lambda \) crosses \(\lambda _*\). To prove the transversality condition of transcritical bifurcation, we use standard notations in Perko (1996). The system (22) can be written in compact form as
and the Jacobian matrix in (23) is denoted as \(A\,=\,J\left( E_2\right) \). For \(\lambda \,=\,\lambda _*\), the matrix A has a zero eigenvalue, eigenvectors corresponding to zero eigenvalue for the matrices A and \(A^t\) are given by
Partial differentiation of \(\mathbf{f}\) with respect to \(\lambda \) gives
and the Jacobian of \(\mathbf{f}_\lambda \) can be calculated as follows:
Hence, we can verify the transversality conditions for transcritical bifurcation as follows:
These ensure that the boundary equilibrium point \(E_2\) loses stability through transcritical bifurcation.
Appendix C
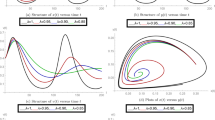
For the values in Table 1, we get the four roots of Eq. (15) as: \(- \,0.0664\), 0.0360 and \(- \,0.0108 \pm 0.0430i\). We get one pair of complex root and the positive root as 0.0360. Here, \(\omega _4 = - 4.6897\times 10^{-6}<0\). According to Lemma 1, Eq. (15) will have at least one positive root.
Equation (16) has three roots: \(- 0.0374\) and \( - 0.0008 \pm 0.0073i\). We have got \(\delta = 2.6996 \times 10^{-11}>0,\)\(H(l)<0\). According to Lemma 2, Eq. (15) has one positive root. Then, Eq. (11) has a pair of purely imaginary root. Finally, we have obtained \(\theta _0= 0.1897\) from (19), as well as the critical value of \(\tau _1\), namely \(\tau _1^*=15.38\), from relation (18).
Rights and permissions
About this article
Cite this article
Al Basir, F., Adhurya, S., Banerjee, M. et al. Modelling the Effect of Incubation and Latent Periods on the Dynamics of Vector-Borne Plant Viral Diseases. Bull Math Biol 82, 94 (2020). https://doi.org/10.1007/s11538-020-00767-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00767-2