Abstract
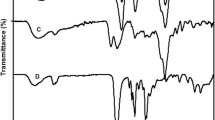
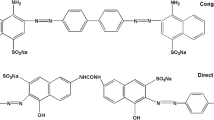
A novel biosorbent yttrium/sodium alginate (Y/SA) hydrogel was prepared by the crosslinking of sodium alginate (SA) and yttrium (Y) ions and characterized by Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS), X-ray Diffraction (XRD), Fourier Transform-Infrared (FTIR), UV visible diffuse reflectance spectroscopy (UV–Vis DRS) and Thermogravimetric Analysis (TGA), respectively. The important parameters affecting the adsorption of Congo Red (CR) and Weak Acid Brilliant Blue RAW (WABB RAW) onto Y/SA were investigated systematically, respectively. The results show that Y/SA gel beads have super-strong adsorption performance for CR and WABB RAW dyes in a wide pH range of 2.0–10.0. The equilibrium data fitted by Langmuir and Freundlich models were found to completely follow Langmuir model where the obtained maximum adsorption capacities of 1567 and 1087 mg/g for CR and WABB RAW were very close to the experimental adsorption capacities, respectively, showing the adsorption of monomolecular layer. The adsorption kinetic data of two dyes by Y/SA fully accorded with the pseudo-second-order rate equation and adsorption processes were mainly controlled by intraparticle diffusion. The results of thermodynamic study indicate the feasible, spontaneous and exothermic nature of adsorption reaction. The further study revealed that electrostatic adsorption, hydrogen bonding and ion exchange reaction were the predominant adsorption for dye removal from aqueous solution by Y/SA gel beads. As a highly efficient, green and conducive to solid–liquid separation biosorbent, Y/SA hydrogel has great potential-application prospects for the purification of high-concentration dyestuff effluent.








Similar content being viewed by others
References
Tharaneedhar V, Senthil Kumar P, Saravanan A, Ravikumar C, Jaikumar V (2017) Sustain Mater Technol 11:1
Anitha T, Senthil Kumar P, Sathish Kumar K (2016) J Water Process Eng 13:127
Waheed A, Mansha M, Kazi IW, Ullah N (2019) J Hazard Mater 369:528
Senthil Kumar P, Ramalingam S (2013) Int J Ind Chem 4:17
Sienkiewicz A, Kierys A, Goworek J (2019) J Disper Sci Technol 40:1396
Grace Pavithra K, Senthil Kumar P, Jaikumar V, Sundar Rajan P (2019) J Ind Eng Chem 75:1
Rashidi NA, Yusup S (2017) Chem Eng J 314:277
Hadi P, Xua M, Ning C, Lin CSK, McKay G (2015) Chem Eng J 260:895
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Sustain Mater Technol 9:10
Liu QM, Li YY, Chen HF, Lu J, Yu GS, Möslang M, Zhou YB (2020) J Hazard Mater 382: https://doi.org/10.1016/j.jhazmat.2019.121040
Zhang L, Sellaoui L, Franco D, Dotto GL, Bajahzar A, Belmabrouk H, Bonilla-Petriciolet A, Oliveira MLS, Li Z (2019) Chem Eng J 372: https://doi.org/10.1016/j.cej.2019.122952
Senthil Kumar P, Sivaranjanee R, Vinothini U, Raghavi M, Rajasekar K, Ramakrishnan K (2013) Desalin Water Treat 51:1
Cho E, Kim J, Park CW, Lee K-W, Lee TS (2018) J Hazard Mater 360:243
Eskhan A, Banat F (2018) J Polym Environ 26:2901
Chiew CSC, Poh PE, Pasbakhsh P, Tey BT, Yeoh HK, Chan ES (2014) Appl Clay Sci 101:444
Agnihotri S, Singhal R (2019) J Polym Environ 27:372
Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed BH (2015) Chem Eng J 270:621
Kumar M, Dosanjh HS, Singh H (2018) J Inorg Organomet P 28: 1688
Xu QY, Chen ZB, Wu ZS, Xu F, Yang DX, He Q, Li G, Chen Y (2019) Bioresour Technol 289:1
Zhang SY, Lyu Y, Su XS, Bian YY, Yu BW, Zhang YL (2016) Environ Earth Sci 75:401
He JS, Cui AN, Ni F, Deng SH, Shen F, Yang G (2018) J Colloid Interface Sci 531:37
Fabryanty R, Valencia C, Soetaredjo FE, Putro JN, Santoso SP, Kurniawan A, Ju YH, Ismadji S (2017) J Environ Chem Eng 5:5677
Wei L, Hong TQ, Liu HB, Chen TH (2017) J Cryst Growth 113:60
Cestari AR, Vieira EFS, Santos AGP, Mota JA, Almeida VP (2004) J Colloid Interface Sci 280:380
Lagergren S (1898) K Sven Vetenskapsakad Handl 24:1
Ho YS, McKay G (1999) Process Biochem 34:451
Weber JW, Morris JC (1963) J Sanit Eng Div 89:31
Jothirani R, Senthil Kumar P, Saravanan A, Narayan Abishek S, Abhishek D (2016) J Ind Eng Chem 39:162
Dotto GL, Pinto LAA (2011) J Hazard Mater 187:164
Zheng Y, Zhu B, Chen H, You W, Jiang C, Yu J (2017) J Colloid Interface Sci 504:688
Zhong L, Tang A, Yan P, Wang J, Wang Q, Wen X, Cui Y (2019) J Colloid Interface Sci 537:450
Lorenc-Grabowska E, Gryglewicz G (2007) Dyes Pigm 74:34
Golder A, Samanta A, Ray S (2006) Chem Eng J 122:107
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Chem Eng J 148:354
Namasivayam C, Arasi D (1997) Chemosphere 34:401
Wang L, Wang A (2007) J Hazard Mater 147:979
Dawood S, Sen TK (2012) Water Res 46:1933
Sayǧili H, Güzel F (2015) Chem Eng Res Des 100:27
Dawood S, Sen TK, Chi P (2014) Water Air Soil Pollut 225:1818
Li J, Ng DHL, Song P, Kong C, Song Y, Yang P (2015) Biomass Bioenerg 75:189
Lei C, Zhu X, Zhu B, Jiang C, Le Y, Yu J (2017) J Hazard Mater 321:801
Tellinghuisen J (2006) Biophys Chem 120:114
Acknowledgements
This study was funded by the National Natural Science Foundation of China (21167011), the Inner Mongolia Natural Science Foundation (2015MS0226) and the Inner Mongolia Normal University Science Research Foundation (112129K18ZZYF006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, B., Yin, H. Superior Adsorption Property of a Novel Green Biosorbent Yttrium/Alginate Gel Beads for Dyes from Aqueous Solution. J Polym Environ 28, 2137–2148 (2020). https://doi.org/10.1007/s10924-020-01757-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01757-0