Abstract
Ectothermic animals are especially susceptible to temperature change, considering that their metabolism and core temperature are linked to the environmental temperature. As global water temperatures continue to increase, so does the need to understand the capacity of organisms to tolerate change. Sheepshead minnows (Cyprinodon variegatus) are the most eurythermic fish species known to date and can tolerate a wide range of environmental temperatures from − 1.9 to 43.0 °C. But little is known about the physiological adjustments that occur when these fish are subjected to acute thermal challenges and long-term thermal acclimation. Minnows were acclimated to 10, 21, or 32 °C for 4 weeks or acutely exposed to 10 and 32 °C and then assessed for swimming performance [maximum sustained swimming velocity (Ucrit), optimum swimming velocity (Uopt)] and metabolic endpoints (extrapolated standard and maximum metabolic rate [SMR, MMR), absolute aerobic scope (AS), and cost of transport (COT)]. Our findings show that the duration of thermal exposure (acute vs. acclimation) did not influence swimming performance. Rather, swimming performance was influenced by the exposure temperature. Swimming performance was statistically similar in fish exposed to 21 or 32 °C (approximately 7.0 BL s−1), but was drastically reduced in fish exposed to 10 °C (approximately 2.0 BL s−1), resulting in a left-skewed performance curve. There was no difference in metabolic end points between fish acutely exposed or acclimated to 10 °C. However, a different pattern was observed in fish exposed to 32 °C. MMR was similar between acutely exposed or acclimated fish, but acclimated fish had a 50% reduction in extrapolated SMR, which increased AS by 25%. However, this enhanced AS was not associated with changes in swimming performance, which opposes the oxygen-capacity limited thermal tolerance concept. Our findings suggest that sheepshead minnows may utilize two distinct acclimation strategies, resulting in different swimming performance and metabolic patterns observed between 10 and 32 °C exposures.
Similar content being viewed by others
Introduction
Estuaries are variable environments that not only transition freshwater to marine habitats, but also link aquatic ecosystems with terrestrial ecosystems. For organisms to survive and inhabit this dynamic aquatic environment, they must either have a capacity for physiological plasticity or possess a broad physiological response. Both of these strategies are paramount for coping with unpredictable and rapid variations in salinity, temperature, and oxygen availability. Estuarine fish are aquatic ectotherms, and as such, their core temperature is contingent on the environmental temperature. Therefore, they are sensitive to changes in temperature, which overall affects enzymatic properties, reaction rates, membrane fluidity, and metabolism (Hochachka and Somero 2002). Within the context of global rising temperatures, some aquatic ectotherms may be particularly vulnerable to temperature change. For example, the Antarctic icefish is an extreme stenotherm (narrow thermal tolerance) that evolved in a relatively stable environment for the last 10–14 million years (Sidell and O’Brien 2006; Somero 2010). Similarly, eurythermic (broad thermal tolerance) species that inhabit environments at the top of their thermal range, as is the case for tropical species, are predicted to be highly susceptible (Nguyen et al. 2011; Somero 2010). Consequently, identifying species that will survive as temperatures continue to rise (Somero 2010) can be used to determine what phenotypic traits are beneficial for thermal survival (Schnell and Seebacher 2008). This information can be used to develop predictive models that could hopefully enact policy change (Clark et al. 2013).
The metabolic theory of ecology proposes that metabolic rate and its plasticity provide the overall basis for multiple ecological phenomena such as migration, species distribution, and species or population responses to climate change (Brown et al. 2004; Duarte 2007). A hypothesis put forward by Pörtner (2001), called the oxygen and capacity limited thermal tolerance (OCLTT) hypothesis, states that oxygen availability (aerobic scope) and capacity (delivery to metabolically active tissues) sets the thermal tolerance of an organism and that this is particularly noticeable in aquatic ectotherms (e.g., fish and invertebrates). Lee et al. (2003) found this pattern in three different stocks of sockeye salmon (Oncorhynchus nerka) and one stock of coho salmon (Oncorhynchus kisutch) that were acclimated at a range of temperatures that mimicked natal river temperatures (5–20 °C). However, it was recently suggested that eurythermic coastal and estuarine species have a left-skewed aerobic scope and swimming performance rather than a bell-shaped curve (Norin et al. 2014). This pattern was observed when the barramundi (Lates calcarifer), an estuarine and eurythermic species, were acclimated at the species preferred temperature (29 °C) and at CTmax (38 °C). It was later suggested that a bell-shaped curve might be typical of stenothermic species, while a left-skewed performance curve may be more representative of eurythermic species (Ern et al. 2014). Within this established framework, we investigated the swimming performance and metabolism of an estuarine species that possesses one of the highest thermal tolerance ranges in teleost fishes.
Sheepshead minnows (Cyprinodon variegatus) are exceptionally tolerant of environmental stressors and occupy various estuarine habitats where salinities range from 0 ppt (fresh) to upward of 45 ppt (hypersaline) (Johnson 1974), and where temperatures range from − 1.9 to 43 °C (Bennett and Beitinger 1997; Harrington and Harrington 1982; Moore 1976). Their broad tolerance ranges are likely related to their extensive habitat range along the Atlantic coast and the Gulf of Mexico, with populations as far north as Cape Cod, MA, and as far west as the Yucatan Peninsula in Mexico (Raimondo et al. 2013). As a species, sheepshead minnows possess the largest thermal tolerance range with a recorded CTmin and CTmax of 0.6 and 45.1 °C, respectively, which is largely consistent with temperatures they encounter in their geographical range (Bennett and Beitinger 1997). For perspective, the thermal breadth of this species spans environmental temperatures that are inhabited by the coldest teleost (Antarctic icefish, − 2 °C) (Franklin et al. 2007) to the warmest teleost (Lake Magadi cichlid, 45 °C) (Farrell 2009). Most of the knowledge about the thermal tolerance of sheepshead minnows have been inferred through the geographical range or from field reports related to temperature fluctuations and fish kills (Moore 1976). Therefore, our goal was to quantify the physiological changes that occur following acclimation and to distinguish between an acute response to temperature and an acclimatory change in the sheepshead minnow.
Methodology
Animals
Adult, mix-sexed sheepshead minnows (C. variegatus; average mass: 2.9 ± 0.4 g; caudal length: 4.2 ± 0.2 cm) were purchased from Aquatic BioSystems, Inc. (Ft. Collins, CO) and shipped overnight to the University of North Texas. The broodstock at Aquatic BioSystems comprised laboratory-raised and wild stock populations, and the last introduction to the broodstock was in 2014 from a wild population sourced at Cape May, NJ (personal communication, Scott Kellman, Owner). Minnows were raised, reared, and maintained at a temperature range of 21–25 °C at this facility (personal communication, Scott Kellman, Owner). At the University of North Texas, fish were housed in 150 l tanks filled with 25 ppt saltwater maintained at ambient room temperature (approximately 21 °C). Saltwater was made by mixing deionized facility water with Instant Ocean® Sea Salt (United Pet Group, Blacksburg, VA). Fish were kept on a 14 h light:10 h dark photoperiod and fed pellet food (Skretting, Tooele, UT) ad libitum every other day. Water quality parameters [pH (7.2–7.6), ammonia (< 5 ppm), salinity (25 ppt), temperature, and dissolved oxygen] (Table 1) were monitored daily, and if levels exceeded these limits, 50% of the aquarium water was changed. Sheepshead minnows were given at least 1 week to recover from handling stress before being transferred to the experimental tanks. All study procedures were approved by the University of North Texas animal care and use committee (IACUC protocol # 18-015).
Chronic thermal acclimation
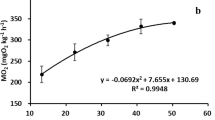
Minnows were randomly assigned one of three 15 l experimental tanks maintained at 25 ppt salinity; saltwater was prepared as above. Experimental tanks were insulated with 2 cm-thick Styrofoam® to prevent temperature fluctuations during the 4-week thermal acclimation period. Water temperature was monitored using a Lauda system temperature controller (Lauda-Brinkman LP, Delran, NJ) and continuously monitored by a submerged thermocouple connected to a MacLab 8e PowerLab (ADInstruments, Sydney, Australia) that was connected to a MAC computer and recorded using LabChart software v 4.1.1. (ADInstruments, Sydney, Australia). In two of the three tanks, the temperature was gradually changed (1 °C daily) from 21 °C to target temperatures of either 10 or 32 °C. Once the target temperature was reached, the aquaria were maintained at the target temperature for 4 weeks (28 days). The target temperatures of 10, 21, and 32 °C were selected because they fall within the broad range of temperatures recorded from native Texas estuaries (Fig. 1 ; NOAA NERRS at http://cdmo.baruch.sc.edu) and are thus ecologically relevant. During the acclimation period, fish were fed pellet food (Skretting, Tooele, UT) ad libitum every other day and fasted 24 h before experimentation. Water quality parameters were measured as above.
Average monthly water temperature (°C) recorded for Mission-Aransas National Estuarine Research Reserve in Port Aransas, Texas from January 2014 to December 2016. Data were sourced from NOAA NERRS at http://cdmo.baruch.sc.edu. Values are presented as averages ± SD
Calibration of swim tunnel respirometer
A single fish (n = 8 per temperature) was placed in a Blazka-style swim respirometer (Loligo Systems, Denmark) to assess Ucrit (maximum sustained swimming speed), extrapolated standard metabolic rate (SMR), extrapolated maximum metabolic rate (MMR), absolute aerobic scope (delta metabolic rate), cost of transport (COT) at Ucrit, and COT at Uopt (optimal swim speed for minimal COT) using automated, intermittent flow respirometry. The following methodology was based on previously published studies from Mager et al. (2014) and Stieglitz et al. (2016). Oxygen consumption inside the swim chamber was recorded using a fiber-optic probe connected to a Witrox4 oxygen meter (Loligo Systems, Denmark). The oxygen meter was calibrated before each swimming trial at 100% air saturation (achieved via submerged air stones) and at 0% saturation (achieved by a 10 g per l Na2SO3 solution). All data were collected using AutoResp2 software (v 2.1.2; Loligo Systems, Denmark).
Animal respiration and swimming performance of thermally acclimated fish
Swimming trials were performed individually using a Blazka-style swim respirometer (Loligo Systems, Denmark) with water temperature set at the respective acclimation temperature (10, 21, or 32 °C) and maintained using a Lauda system temperature controller (Lauda-Brinkman LP, Delran, NJ). All fish were left to habituate at a minimum of 4 h at 0.5 body lengths (BL) s−1 or 0.25 BL s−1 for 10 °C acclimated fish since preliminary overnight trials (n = 4) indicated this was sufficient time to reach a stable routine metabolic rate (RMR). A stable RMR was determined by plotting oxygen consumption over time until there was no change in oxygen consumption over three consecutive points (i.e., the slope was equal to zero). For fish acclimated to 21 and 32 °C, swimming speed was increased by 0.5 BL s−1 at 20 min intervals. For fish acclimated to 10 °C, swimming speed was increased by 0.25 BL s−1 at 20 min intervals. In the initial trials (n = 4), there was no difference in Ucrit of 10 °C acclimated fish exercised at 0.5 BL s−1 intervals compared to 10 °C acclimated fish exercised at 0.25 BL s−1 intervals (2.65 ± 0.25 and 2.74 ± 0.35 BL s−1 respectively). Therefore, we decreased the rate at which the swimming speed would be increased to increase the metabolic sampling points for extrapolating SMR and MMR. Food was withheld from all fish for a 24 h period before introduction to the swim chamber to prevent confounding effects of digestion on swimming performance and waste accumulation. Swimming trials were terminated when the fish became fatigued and could not be induced to continue swimming. Fatigue was determined when the fish no longer maintained their position in the chamber and rested along the back screen. Following termination of the trial, fish were euthanized with a lethal dose of buffered MS-222 to collect blood samples and to record organ metrics (mass and lengths). Mass and body length of each fish were collected post-swimming to reduce handling stress prior to recording RMR.
Animal respiration and swimming performance of fish acutely exposed to temperature
Individual sheepshead minnows (n = 8 per temperature change) from acclimation tanks maintained at 21 °C were transferred to the swim tunnel respirometer maintained at 21 °C and left to habituate overnight where RMR was recorded. The water temperature was rapidly decreased or increased to the target temperature at a rate of 2 °C per hour. Preliminary data suggested that a temperature rate of change faster than 2 °C per hour resulted in 100% mortality upon reaching 32 °C (n = 3). After a stable RMR was established following the temperature change, swimming trials were performed as described above. Upon completion, fish were euthanized with a lethal dose of buffered MS-222 to collect blood samples. Mass and body length of each fish were collected post-swimming to reduce handling stress prior to recording routine metabolic rate.
Standardization and background respiration
After the fish was removed from the swim chamber, the swim chamber was reassembled, and oxygen consumption was recorded for an hour to determine background oxygen consumption. At the end of every trial, the swim chamber was cleaned with a 10% bleach solution, triple rinsed with DI water, and allowed to dry overnight. It should be mentioned that while there were different habituation times between acclimation (4 h at acclimation temperature) and acute exposures (12 h at 21 °C), all swimming trials began at the same time of day (10:00 AM CST). Additionally, the values recorded for RMR between acute and acclimated fish of the same temperature were not different from each other. Lastly, background respiration in the swim tunnel once the fish was removed was negligible (< 10 mg O2 kg−1 h−1) regardless of the amount of time spent in the swim chamber.
Calculations and statistical analysis
For each fish, the logarithm of mass-specific MO2 was plotted against swimming velocity and fit to a least-squares standard regression model, an exponential regression model, and a logarithmic model; we chose the least-squares regression model because it resulted in the highest R2 value (0.93 ± 0.01) and best modeled (72%) the dataset. Data from trials were not used in the final analysis if the regression R2 was below 0.85. Data were not allometrically scaled because there was no correlation between metabolic rate and mass or temperature (supplemental Fig 1 and supplemental Table 1). The resulting equation was used to solve for SMR (y-intercept) and MMR (extrapolated at Ucrit). Ucrit was expressed in BL s−1 and calculated using the following equation:
where Uf is the highest swim velocity maintained for a complete interval (cm s−1), T is the time spent in the final interval (s), t is the total time of the interval (s), and dU is the increment in swim speed (cm s−1). In this study, COT is defined as the energetic expenditure of movement over a distance and is expressed as mg O2 consumed per kg fish per meter traveled. COT was calculated by dividing MO2 by swimming velocity at each interval, which resulted in a parabola-shaped plot that was fit to a second-order polynomial regression model (Table 2). The resulting model and formula were used to calculate (1) COT at Ucrit, (2) (Uopt), and (3) COT at Uopt.
(1) where a is equal to the slope, b is equal to the relative minimum, c is equal to the relative maximum, and x is equal to Ucrit. (2) Uopt was calculated by setting the first derivative of the above equation to zero and solving for x to find the vertex of the parabola. (3) COT at Uopt was calculated using the above equation except x is now equal to the value of the vertex of the parabola or Uopt, which calculates the absolute minimum of the parabola or COT at Uopt.
All statistical tests were performed using Statistica Version 13.3. Data were tested for normality and homogenous variances, and if assumptions were met, then data were tested using parametric tests. For each measured variable (SMR, MMR, AS, Ucrit, Uopt, \( {\text{COT}}_{{U_{\text{crit}} }} \), \( {\text{COT}}_{{U_{\text{opt}} }} \)), a repeated-measures GLM was used with temperature (10 °C, 21 °C, and 32 °C), length of exposure (acute and acclimation), and sex as explanatory variables, as well as the interaction between these variables. Significant differences were highlighted using dissimilar letters.
Results
Water quality parameters and animal metrics
Table 1 summarizes the results of all water quality parameters that were monitored (temperature, dissolved oxygen, salinity, pH, and ammonia). During the acclimation period, females that became gravid were removed from the final analysis because they had statistically different metabolic rates and swimming performance than non-gravid females and males (data not shown). There was no difference in the metabolic rate (p = 0.9) or swimming performance (p = 0.2) between adult males and non-gravid females, and the final distribution of each sex per acclimation temperature is depicted in Table 3. Lastly, a limitation of the study is that acclimation exposures were only replicated once (i.e., all eight fish in one acclimation tank per temperature), which could be considered a form of psuedoreplication. However, this is less of a concern for the acute exposures that were replicated eight times individually per temperature within the swim chamber.
Thermal acclimation influences on blood parameters post-exercise
Hematocrit from 21 °C acclimated fish post-exercise was 45.9 ± 5.3% and was reduced by nearly 60% following thermal acclimation to 10 and 32 °C (Fig. 2). Hemoglobin concentration was highest in 10 °C acclimated fish at 13.7 ± 0.9 g dl−1 and was not significantly different from fish acclimated at 21 °C (Fig. 2). However, fish acclimated at 32 °C had hemoglobin concentrations that were drastically reduced compared to fish acclimated at colder temperatures (Fig. 2).
Measurements of post-exercise hematocrit (left) and hemoglobin concentration (right) from sheepsheads minnows that were acclimated to 10 °C (white bar), 21 °C (gray bar), or 32 °C (black bar). Data are represented as averages ± SE. Dissimilar letters designate significance across temperatures (p < 0.05)
Metabolic responses to temperature acclimation or acute thermal exposure
SMR of 21 °C acclimated fish was 336.94 ± 40.84 mg O2 h−1 kg−1 and was slightly lower but not statistically different from SMR recorded for 32 °C acclimated fish (460.23 ± 40.45 mg O2 h−1 kg−1; Fig. 3). SMR in 10 °C acclimated fish was reduced by approximately 50% compared to fish acclimated to warmer temperatures (Fig. 3). There was no significant difference in SMR between 10 °C acclimated and acutely exposed fish (Fig. 3). SMR of fish acutely exposed to 32 °C was approximately twofold higher than SMR of fish acclimated to the same temperature (Fig. 3).
Standard metabolic rate (SMR; top), maximum metabolic rate (MMR; middle), and aerobic scope (AS; bottom) of sheepsheads minnows that were either acclimated to 10, 21, or 32 °C (closed bars; left) or acutely exposed to 10 or 32 °C (open bars; right). Data are represented as averages ± SE. Dissimilar letters designate significant differences between temperature and length of exposure (p < 0.05)
MMR of 21 °C acclimated fish was 920.09 ± 62.86 mg O2 h−1 kg−1 (Fig. 3). Compared to this group, the MMR of 10 °C acclimated fish was reduced by 67% and the MMR of 32 °C acclimated fish was increased by 33% (Fig. 3). However, there were no differences in MMR of fish that were acutely exposed or acclimated to temperatures of 10 °C or 32 °C (Fig. 3).
Absolute AS of fish acclimated to 21 °C was 583.15 ± 100.94 mg O2 h−1 kg−1, which is approximately 2.5-fold higher than the 10 °C exposed (acutely or acclimated) fish (Fig. 3). The AS of fish acutely exposed to 32 °C and fish acclimated to 21 °C were similar; however when fish were acclimated to 32 °C, AS significantly increased by 25% (Fig. 3). Log metabolic rates (both SMR and MMR) were each plotted against the log mass of individual fish and sorted by temperature and exposure (supplemental Fig. 1), and there was no correlation with mass and metabolic rate regarding temperature (supplemental Table 1).
Acclimation or acute thermal exposure effects on swimming performance
The Ucrit of 21 °C acclimated fish was 6.6 ± 0.3 BL s−1 and not statistically different from the swimming velocity of 32 °C acclimated fish at 7.2 ± 0.5 BL s−1 (Fig. 4). Ucrit in 10 °C acclimated fish was reduced threefold with an average swimming speed of 2.4 ± 0.1 BL s−1 (Fig. 4). Uopt of 21 °C acclimated fish was 4.5 ± 0.3 BL s−1 which was approximately 50% higher than fish acclimated at 10 °C and 25% lower than fish acclimated at 32 °C (Fig. 4). At any given temperature, there was no difference between acute or acclimation exposure for both Ucrit and Uopt (Fig. 4). Regardless of the exposure temperature or length of exposure, Uopt was on average 30% lower than Ucrit (Fig. 4).
Maximal sustained swimming velocity (Ucrit; top) and optimal swimming velocity (Uopt; bottom) of sheepsheads minnows that were either acclimated to 10, 21, or 32 °C (closed bars; left) or acutely exposed to 10 or 32 °C (open bars; right). Data are represented as averages ± SE. Dissimilar letters designate significant differences between temperature and length of exposure (p < 0.05)
The COT at Ucrit (\( {\text{COT}}_{{U_{\text{crit}} }} \)) in 21 °C acclimated fish was 1.17 ± 0.08 mg O2 kg−1 m−1 and was not different from the COT in both 10 °C and 32 °C acclimated fish (Fig. 5, 6). There were no differences in \( {\text{COT}}_{{U_{\text{crit}} }} \) between fish acutely exposed to or acclimated at 10 °C (Figs. 5, 6). However, fish acutely exposed to 32 °C possessed slightly higher \( {\text{COT}}_{{U_{\text{crit}} }} \) than fish acclimated to 32 °C (Figs. 5, 6), even though they had the same swimming velocity (Fig. 4).
Cost of transport at maximal sustained swimming velocity (COT Ucrit; top) and the cost of transport at optimal swimming velocity (COT Uopt; bottom) of sheepsheads minnows that were either acclimated to 10, 21, or 32 °C (closed bars; left) or acutely exposed to 10 or 32 °C (open bars; right). Data are represented as averages ± SE. Dissimilar letters designate significant differences between temperature and length of exposure (p < 0.05)
Cost of transport plotted against swimming speed for sheepsheads minnows that were either acclimated to 10 (white circles), 21 (gray circles), or 32 °C (black circles) or acutely exposed to 10 (white triangles) or 32 °C (black triangles). Each data point represents the cost of transport (averages ± SE) at intervals of 0.5 body lengths per second, starting at 1.0 body lengths per second. Dotted lines represent the average COT across all
COT at Uopt (\( {\text{COT}}_{{U_{\text{opt}} }} \)) for 21 °C acclimated fish was 0.72 ± 0.05 mg O2 kg−1 m−1 and was similar to \( {\text{COT}}_{{U_{\text{opt}} }} \) for both fish acclimated at or acutely exposed to 10 °C (Figs. 5 and 6). \( {\text{COT}}_{{U_{\text{opt}} }} \) for 32 °C acclimated fish increased by 25% compared to fish acclimated at colder temperatures (Figs. 5, 6). Fish that were acutely exposed to 32 °C had slightly higher \( {\text{COT}}_{{U_{\text{opt}} }} \) compared to fish that were acclimated to 32 °C (Figs. 5, 6).
Discussion
Fluctuation in environmental temperature has a profound effect on the physiology of aquatic ectotherms. Despite this truism, many questions remain regarding the effects of both acute and chronic temperature variations in many fish species. The main objectives of our study were to (1) determine the effects of temperature on hematological parameters (as a proxy for oxygen-carrying capacity), metabolic rate, and swimming performance in the eurythermic sheepshead minnow and (2) compare responses between different rates and duration of temperature exposure (acute versus acclimation). Our results show that there were no differences in SMR, MMR, or the AS whether fishes were acutely exposed (5.5 h) or acclimated (4 weeks) to 10 °C and these parameters were reduced twofold compared to 21 °C acclimated fish (Fig. 3). Fish that were acclimated to 32 °C had a 25% increase in their AS compared to 21 °C acclimated fish, which we attributed to the down-regulation of SMR during acclimation (Fig. 3). Nevertheless, this increase in AS did not enhance the swimming performance of fish acclimated to 32 °C (Fig. 4). Also, swimming performance was significantly reduced when fish were exposed to 10 °C (Fig. 4), even though they had higher blood oxygen-carrying capacity (Fig. 2) compared to the fish acclimated at warmer temperatures. Lastly, we found that exposure length (acute versus acclimation) did not affect swimming performance (Fig. 4).
Post-exercise and thermal acclimation influences on blood parameters
Peterson (1990) found that sheepshead minnows maintained in normoxic conditions (150 mm Hg) at ambient temperature (25 °C) had a hematocrit of approximately 25%, while fish maintained in hypoxic conditions (40 mmHg) had slightly elevated hematocrits at approximately 30%. These values are considerably lower than what was found in our study (21 °C acclimated, post-exercise minnows had hematocrit values of approximately 50%), and we speculated that the elevated levels were because our samples were taken post-exercise. This suggests that fish acclimated at 21 °C have the capacity to regulate hematocrit during exercise, which in turn would increase the oxygen-carrying capacity of the blood but at the cost of increased blood viscosity (Birchard 1997). This trade-off could explain the reduction in hematocrit in 32 °C acclimated fish, where the work to pump viscous blood is potentially not energetically favorable even with increased oxygen-carrying capacity (Crowell et al. 1959).
Another critical component of oxygen transport is overall hemoglobin content in the erythrocytes. Our findings show that hemoglobin concentration was highest in 10 °C acclimated fish at approximately 13.7 g dl−1 (Fig. 2) or 2.12 mM (assuming the molecular weight of hemoglobin is equal to 64,550 g mol−1) (Billett 1990). This value is consistent with values found previously in highly active, migratory marine species belonging to the Scombridae family: Sarda sarda at 2.39 ± 0.20 mM, Scomber japonicas at 2.30 ± 0.16 mM, and Scombermorus brasiliensis at 1.92 ± 0.40 mM (Wilhelm Filho et al. 1992). After acclimation to 32 °C and exercise, hemoglobin concentration was reduced in fish by 62% at 5.4 ± 0.7 g dl−1 (Fig. 2) or 0.84 mM. However, these hemoglobin concentrations are found in sedentary, non-migratory marine teleost species (Urophycis brasilensis at 0.83 ± 0.16 mM); Wilhelm Filho et al. 1992). Therefore, while 32 °C acclimation does reduce the hemoglobin concentration in erythrocytes, it is not a reduction that appears outside of levels typically found in teleost fish. Increasing hemoglobin concentration may be a mechanism to increase the oxygen-carrying capacity of the blood, bypassing the consequences of increased blood viscosity (e.g., increased cardiac work). However, at this time, the authors are unsure of the purpose or benefits of having increased hemoglobin concentration per volume when exposed to colder temperatures rather than warmer temperatures.
Metabolic responses to temperature acclimation or acute thermal exposure
Overall, when sheepshead minnows were exposed to 10 °C, SMR, MMR, and AS were reduced compared to minnows acclimated at 21 °C (Fig. 3). There was no difference in these metabolic end points whether the fish was acutely exposed to 10 °C or acclimated to 10 °C. This indicates that during acclimation to 10 °C, there was no compensatory metabolic change to overcome the thermodynamic effects of colder temperatures in the sheepshead minnow. Our data are consistent with what was previously found in an ecologically similar species, the Atlantic killifish (Fundulus heteroclitus), in which RMR and MMR did not change during a 6-week acclimation period, and there was no physiological compensation associated with cold acclimation (Healy et al. 2017). Similarly to the sheepshead minnow, there were no differences in the AS of killifish acclimated and acutely exposed to 5, 10, and 15 °C (Healy and Schulte 2012).
Generally, metabolic rate increases with increasing temperatures. However, when sheepshead minnows were acclimated at 32 °C or acutely exposed to 32 °C, there was no change in MMR, but SMR was significantly reduced following acclimation to 32 °C (Fig. 3). A recent study conducted with Icelandic sticklebacks (Gasterosteus aculeatus) from warmer and colder habitats measured at a common temperature reported that fish from the warmer habitats had significantly depressed SMR compared to fish from colder habitats (Pilakouta et al. 2020). This pattern was similarly observed in shorthorn sculpin (Myoxocephalus Scorpius) acclimated to 16 °C over 8 weeks (i.e., minute changes in MMR and more considerable change in SMR during acclimation) (Sandblom et al. 2014). Again, this pattern was found in a small African cichlid (Pseudocrenilabrus multicolor victoriae) where SMR depression was evident at the highest acclimation temperature (34 °C and MMR changed relatively little between 30 and 34 °C (McDonnell and Chapman 2016). Sheepshead minnows may be undergoing metabolic depression following acclimation at higher temperatures as an energy-saving strategy (i.e., the minimum energy cost for basal function is reduced) to cope with living in unpredictable, estuarine habitats. It was reported by Rummer et al. (2014) that equatorial damselfish inhabiting the Great Barrier Reef could be classified as thermal specialists or thermal generalists. Damselfish species that were thermal specialists had significant increases in MMR and reduced AS, but higher aerobic performance. This was likely because these species evolved in stable and narrow environmental windows, and there would be no evolutionary pressure to drive metabolic compensation during acclimation (Rummer et al. 2014). The authors speculated that within these damselfish, there is an evolutionary trade-off where some species lose aerobic performance to save energy at rest, while others may incur higher energetic costs at rest but with better aerobic performance (Rummer et al. 2014). Concerning the sheepshead minnow, which is a thermal generalist, the reduction in SMR at higher temperatures could be crucial to reduce energy expenditure during unpredictable temperature shifts to survive until favorable conditions return. As a species, sheepshead minnows are not known to migrate long distances and instead display strong site fidelity (Chitty and Able, 2004). Considering that they frequently inhabit isolated tidal pools that are warmed by the summer sun to temperatures of up to 43 °C (Bennett and Beitinger 1997), reducing SMR to enhance survival seems likely.
Additionally, sheepshead minnows acclimated at 32 °C possessed a 25% increase in AS compared to minnows that were acutely exposed to 32 °C, which we attributed to the observed down-regulation of SMR following acclimation to 32 °C. We observed in the sheepshead minnows that AS increased as acclimation temperature increased, which was previously recorded in the Atlantic halibut (Hippoglossus hippoglossus) as temperatures approached CTmax (Gräns et al. 2014). However, in an ecologically similar species such as the Atlantic killifish, AS increased until 30 °C and then decreased as acclimation temperature approached CTmax (Healy and Schulte 2012; Fangue et al. 2006). Further metabolic studies need to be conducted for the sheepshead minnow at temperatures closer to their reported CTmin and CTmax to determine the general pattern of AS within this species across its thermal breadth.
Acclimation or acute thermal exposure effects on swimming performance
The only change in Ucrit was observed in minnows acclimated and acutely exposed to 10 °C, which was reduced by approximately 50%. Similarly to our study, Fangue et al. (2008) found that swimming performance (Ucrit) changed very little over acclimation temperatures between 10 and 32 °C, but was reduced nearly 50% after 5 °C acclimation in killifish. It was previously reported that during cold acclimation, killifish do not change myosin isoform expression, exhibit minimal increases in myofibril ATPase activity, and only slight decreases in contraction time (Johnson and Bennett 1995). These observations were consistent with their minor change in swimming performance during cold acclimation. By comparison, the common goldfish (Carassius auratus) displayed enhanced swimming performance following cold acclimation likely due to differential myosin isoform expression, enhanced ATPase activity, and a faster rate of contraction (Johnson and Bennett 1995). The mechanism for the reduced Ucrit in sheepshead minnows following cold acclimation is likely similar to the killifish, but this possibility has not been explored. Additionally, Fangue et al. (2008) found a 65% decrease in swimming performance of killifish that were acutely exposed to 5 °C, compared to only a 50% decrease in swimming performance of killifish that were acclimated at 5 °C. However, this is in sharp contrast to our study, where we saw no change in swimming performance between acute and acclimation exposure at 10 °C in the sheepshead minnow. Similarly, we found no difference in the swimming performance (both Ucrit and Uopt) of sheepshead minnows, whether they were acutely exposed or acclimated to 32 °C. Thermal plasticity of skeletal muscle properties (nerve stimulation, contractile kinetics, force generation, and enzyme activity) has evolved independently in several teleost lineages (Johnston and Temple 2002). Perhaps, the skeletal muscle of the sheepshead minnow displays high plasticity when exposed to a wide range of temperatures (which allowed for the extensive habitat range). Likely because of this, we observed no difference in the swimming performance, whether the minnows were acutely exposed or acclimated to a given temperature.
Even though acclimation to 32 °C resulted in an apparent down-regulation of SMR, which translated into a 25% gain in AS, there was no change in Ucrit between 21 and 32 °C acclimated fish (Fig. 4). This has been found in numerous teleost species to date, where AS did not influence overall performance (Clark et al. 2013; Ern et al. 2014; Norin et al. 2014; Gräns et al. 2014). To reiterate, enhanced AS following acclimation at 32 °C did not translate to gains in the aerobic performance of the sheepshead minnow. Not only did acclimation to 32 °C increase AS, but it also reduced the cost of transport at Ucrit compared to minnows that were acutely exposed to the same temperature (Fig. 5). In the European sea bass (Dicentrarchus labarx), it was observed that COT was not influenced by acclimation temperature (Claireaux et al. 2006). However, in the chub mackerel (Scomber japonicas), it was found that COT increased with acclimation temperature (Dickson et al. 2002). In the sheepshead minnow, COT at Ucrit does increase with increasing temperature, but acclimation to 32 °C reduces COT compared to minnows that are acutely exposed to 32 °C. The mechanisms related to these observed species-specific differences in the COT (at or near Ucrit) associated with thermal acclimation have largely been unexplored in the literature (Claireaux et al. 2006). However, it could be the result of species-specific differences in cardiac output regulation, muscle fiber recruitment, contractile properties and kinetics, or mitochondrial energetics.
The following studies provide future research avenues to better understand the changes in swimming performance and metabolism associated with thermal acclimation and acute exposure in a broadly tolerant estuarine fish. Investigating changes in skeletal muscle parameters such as glycogen stores following thermal acclimation (Fangue et al. 2008) would help to understand what mechanistic changes occur that decrease aerobic performance at colder temperatures. A closely related species, the desert pupfish (Cyprinodon macularius), exhibits bouts of paradoxical anaerobism during thermal acclimation to 28–33 °C (Heuton et al. 2018). Therefore, it would be beneficial to determine the relationship between thermal plasticity and reliance on anaerobic respiration. Recently, it was found that sheepshead minnows undergo rapid asymmetrical seasonal acclimatization (i.e., they gain warm tolerance during cold exposure and vice versa) (Fangue et al. 2014), and it would be important to understand how that time course impacts other physiological changes.
To conclude, our study showed there was no metabolic compensation following 10 °C acclimation, but acclimation to 32 °C resulted in SMR suppression without a reciprocal reduction in MMR. The SMR suppression led to enhanced AS but without any benefits conferred to swimming performance, which is in contrast with the OCLTT concept. It appears that these two strategies play a role in surviving such a broad thermal range but at the cost of enhanced performance. Overall, our study adds insight into the differential changes to metabolic and swimming performance that an ectothermic species may undergo as global temperatures continue to rise.
References
Bennett WA, Beitinger TL (1997) Temperature tolerance of the sheepshead minnow, Cyprinodon variegatus. Copeia 1997:77–87. https://doi.org/10.2307/1447842
Billett HH (1990) Hemoglobin and hematocrit. In: Walker HK, Hall WD, Hurst JW (eds) Clinical methods: the history, physical, and laboratory examinations. Butterworth Publishers, a division of Reed Publishing, Boston
Birchard GF (1997) Optimal hematocrit: theory, regulation and implications. Am Zool 37:65–72
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Chitty JD, Able KW (2004) Habitat use, movements and growth of the sheepshead minnow, Cyprinodon variegatus, in a restored salt marsh in Delaware Bay. Bull N J Acad Sci 49:1–9
Claireaux G, Couturier C, Groison A-L (2006) Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J Exp Biol 209:3420–3428
Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216:2771–2782
Crowell JW, Ford RG, Lewis VM (1959) Oxygen transport in hemorrhagic shock as a function of the hematocrit ratio. Am J Physiol-Leg Content 196:1033–1038
Dickson KA, Donley JM, Sepulveda C, Bhoopat L (2002) Effects of temperature on sustained swimming performance and swimming kinematics of the chub mackerel Scomber japonicus. J Exp Biol 205:969–980
Duarte CM (2007) Marine ecology warms up to theory. Trends Ecol Evol 22:331–333
Ern R, Phuong NT, Wang T, Bayley M (2014) Oxygen delivery does not limit thermal tolerance in a tropical eurythermal crustacean. J Exp Biol 217:809–814
Fangue NA, Hofmeister M, Schulte PM (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol 209:2859–2872
Fangue NA, Mandic M, Richards JG, Schulte PM (2008) Swimming performance and energetics as a function of temperature in killifish Fundulus heteroclitus. Physiol Biochem Zool 81:389–401
Fangue NA, Wunderly MA, Dabruzzi TF, Bennett WA (2014) Asymmetric thermal acclimation responses allow sheepshead minnow Cyprinodon variegatus to cope with rapidly changing temperatures. Physiol Biochem Zool 87:805–816
Farrell A (2009) Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J Exp Biol 212:3771–3780
Franklin CE, Davison W, Seebacher F (2007) Antarctic fish can compensate for rising temperatures: thermal acclimation of cardiac performance in Pagothenia borchgrevinki. J Exp Biol 210:3068–3074
Gräns A et al (2014) Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J Exp Biol 217:711–717
Harrington R, Harrington E (1982) Effects on fishes and their forage organisms of impounding a Florida salt marsh to prevent breeding by salt marsh mosquitoes. Bull Mar Sci 32:523–531
Healy TM, Schulte PM (2012) Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic scope in the common killifish (Fundulus heteroclitus). Physiol Biochem Zool 85:107–119. https://doi.org/10.1086/664584
Healy TM, Chung DJ, Crowther KG, Schulte PM (2017) Metabolic and regulatory responses involved in cold acclimation in Atlantic killifish, Fundulus heteroclitus. J Comp Physiol B 187:463–475. https://doi.org/10.1007/s00360-016-1042-9
Heuton M et al (2018) Oxygen consumption of desert pupfish at ecologically relevant temperatures suggests a significant role for anaerobic metabolism. J Comp Physiol B 188:821–830. https://doi.org/10.1007/s00360-018-1174-1
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Biochemistry and Molecular Biology Education. Oxford University Press, New York
Johnson WE (1974) Morphological variation and local distribution of Cyprinodon variegatus in Florida. Retrospective theses and dissertations, 109. https://stars.library.ucf.edu/rtd/109
Johnson T, Bennett A (1995) The thermal acclimation of burst escape performance in fish: an integrated study of molecular and cellular physiology and organismal performance. J Exp Biol 198:2165–2175
Johnston IA, Temple GK (2002) Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J Exp Biol 205:2305–2322
Lee C, Farrell A, Lotto A, MacNutt M, Hinch S, Healey M (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206:3239–3251
Mager EM et al (2014) Acute embryonic or juvenile exposure to Deepwater Horizon crude oil impairs the swimming performance of mahi-mahi (Coryphaena hippurus). Environ Sci Technol 48:7053–7061. https://doi.org/10.1021/es501628k
McDonnell LH, Chapman LJ (2016) Effects of thermal increase on aerobic capacity and swim performance in a tropical inland fish. Comp Biochem Physiol Part A Mol Integr Physiol 199:62–70. https://doi.org/10.1016/j.cbpa.2016.05.018
Moore RH (1976) Observations on fishes killed by cold at Port Aransas, Texas, 11–12 January 1973. Southwestern Nat 20:461–466. https://doi.org/10.2307/3669862
Nguyen KDT, Morley SA, Lai C-H, Clark MS, Tan KS, Bates AE, Peck LS (2011) Upper temperature limits of tropical marine ectotherms: global warming implications. PloS One 6:e29340–e29340. https://doi.org/10.1371/journal.pone.0029340
Norin T, Malte H, Clark TD (2014) Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J Exp Biol 217:244–251. https://doi.org/10.1242/jeb.089755
Peterson MS (1990) Hypoxia-induced physiological changes in two mangrove swamp fishes: sheepshead minnow, Cyprinodon variegatus lacepede and sailfin molly, Poecilia latipinna (lesueur). Comp Biochem Physiol A Physiol 97:17–21. https://doi.org/10.1016/0300-9629(90)90715-5
Pilakouta N, Killen SS, Kristjánsson BK, Skúlason S, Lindström J, Metcalfe NB, Parsons KJ (2020) Multigenerational exposure to elevated temperatures leads to a reduction in standard metabolic rate in the wild. Funct Ecol 34:1205–1214. https://doi.org/10.1111/1365-2435.13538
Pörtner H (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Raimondo S, Rutter H, Hemmer B, Jackson C, Cripe G (2013) The influence of density on adults and juveniles of the estuarine fish, the sheepshead minnow (Cyprinodon variegatus). J Exp Mar Biol Ecol 439:69–75
Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL (2014) Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Change Biol 20:1055–1066. https://doi.org/10.1111/gcb.12455
Sandblom E, Gräns A, Axelsson M, Seth H (2014) Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: implications in a thermally extreme future. Proc R Soc Lond B Biol Sci 281:20141490
Schnell A, Seebacher F (2008) Can phenotypic plasticity facilitate the geographic expansion of the tilapia Oreochromis mossambicus? Physiol Biochem Zool 81:733–742
Sidell BD, O’Brien KM (2006) When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol 209:1791–1802
Somero G (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Stieglitz JD, Mager EM, Hoenig RH, Benetti DD, Grosell M (2016) Impacts of deepwater horizon crude oil exposure on adult mahi-mahi (Coryphaena hippurus) swim performance. Environ Toxicol Chem 35:2613–2622
Wilhelm Filho D, Eble GJ, Kassner G, Caprario FX, Dafré AL, Ohira M (1992) Comparative hematology in marine fish. Comp Biochem Physiol A Physiol 102:311–321
Acknowledgements
The authors would like to thank Dr. Aaron Roberts at the University of North Texas for supplying bench space and aquarium equipment at the UNT Environmental Science Aquatics Facility for the duration of these exposures. We would also like to thank Dr. Kurt Gamperl at Memorial University of Newfoundland for his valuable insight and recommendations during the data analysis and writing process. Lastly, we would like to thank the anonymous reviewers whose constructive feedback helped elevate this paper. This project was made possible through the University of North Texas start-up funds awarded to Dane A. Crossley and Edward M. Mager.
Author information
Authors and Affiliations
Contributions
Conceptualization: ARK and DACII. Methodology: ARK and EMM. Formal analysis and investigation: ARK. Writing—original draft preparation: ARK. Writing—review and editing: ARK, DACII, and EMM. Funding acquisition: DACII, and EMM. Resources: DACII, and EMM. Supervision: DACII, and EMM.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Additional information
Communicated by Bernd Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kirby, A.R., Crossley, D.A. & Mager, E.M. The metabolism and swimming performance of sheepshead minnows (Cyprinodon variegatus) following thermal acclimation or acute thermal exposure. J Comp Physiol B 190, 557–568 (2020). https://doi.org/10.1007/s00360-020-01293-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01293-2