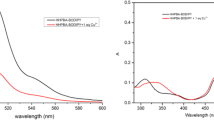
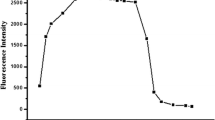
A novel fluorescent probe, 2-allyl-6-((2-((2-hydroxy-5-nitrobenzylidene)amino)ethyl)amino)-1H-benzo[de]isoquinoline- 1,3(2H)-dione (ABID), based on naphthalimide-Schiff base, has been designed and synthesized for the monitoring of Cu2+ ions. In solution (DMSO/HEPES, 1:1, v/v, pH 7.4), ABID displayed fluorescence quenching towards Cu2+ ions over other important metal ions. A good linearity with a correlation coefficient (R2) of 0.99 validated that the ABID probe could be used to detect Cu2+ ions in 0.5–5.0 μM concentrations. The limit of detection of ABID for Cu2+ could reach at 3.4 × 10–7 M level, and the quenching constant (KSV) of ABID towards Cu2+ was calculated to be 3.4 × 104 M–1. The 2:1 stoichiometry and the binding mode between ABID and Cu2+ were studied by a Job plot and UV-Vis and fluorescence titration. Additionally, ABID was successfully employed to monitor Cu2+ in the Yellow River and tap water samples.
Similar content being viewed by others
References
V. B. Bojinov, N. I. Georgiev, and P. S. Nikolov, J. Photochem. Photobiol. A, 193, 129–138 (2008).
Y. M. Yang, Q. Zhao, W. Feng, and F. Y. Li, Chem. Rev., 113, 192–270 (2013).
E. Gaggelli, H. Kozlowski, D. Valensin, and G. Valensin, Chem. Rev., 106, 1995–2044 (2006).
G. L. Millhauser, Acc. Chem. Res., 37, 79–85 (2004).
C. Beyer, U. Böhme, C. Pietzsch, and G. Roewer, J. Organomet. Chem., 654, 187–201 (2002).
E. L. Que, D. W. Domaille, and C. J. Chang, Chem. Rev., 108, 1517–1549 (2008).
R. Zhang, X. J. Yu, Y. J. Yin, Z. Q. Ye, G. L. Wang, and J. L. Yuan, Anal. Chim. Acta, 691, 83–88 (2011).
B. Chen and P. Zhong, Anal. Bioanal. Chem., 381, 986–992 (2005).
Y. L. Xu, S. S. Mao, H. P. Peng, F. Wang, H. Zhang, S. O. Aderinto, and H. L. Wu, J. Lumin., 192, 56–63 (2017).
Y. L. Xu, S. O. Aderinto, H. L. Wu, H. P. Peng, H. Zhang, J. W. Zhang, and X. Y. Fan, Z. Naturforsch. B, 72, 35–41 (2017).
S. O. Aderinto, Y. L. Xu, H. P. Peng, F. Wang, H. L. Wu, and X. Y. Fan, J. Fluoresc., 27, 79–87 (2017).
J. H. Hu, J. B. Li, J. Qi, and Y. Sun, Sensor. Actuat. B: Chem., 208, 581–587 (2015).
W. K. Dong, X. L. Li, L. Wang, Y. Zhang, and Y. J. Ding, Sensor. Actuat. B: Chem., 229, 370–378 (2016).
W. K. Dong, S. F. Akogun, Y. Zhang, Y. X. Sun, and X. Y. Dong, Sensor. Actuat. B: Chem., 238, 723–734 (2017).
H. L. Wu, S. O. Aderinto, Y. L. Xu, H. Zhang, and X. Y. Fan, J. Appl. Spectrosc., 84, 25–30 (2017).
G. Z. Huang, C. Li, X. T. Han, S. O. Aderinto, K. S. Shen, S. S. Mao, and H. L. Wu, Luminescence, 33, 660–669 (2018).
H. P. Peng, K. S. Shen, S. S. Mao, X. K. Shi, Y. L. Xu, S. O. Aderinto, and H. L. Wu, J. Fluoresc., 27, 1191–1200 (2017).
F. Wang, Y. L. Xu, S. O. Aderinto, H. P. Peng, H. Zhang, and H. L. Wu, J. Photochem. Photobiol. A, 332, 273–282 (2017).
K. S. Shen, S. S. Mao, X. K. Shi, F. Wang, Y. L. Xu, S. O. Aderinto, and H. L. Wu, Luminescence, 33, 54–63 (2018).
C. Li, X. T. Han, S. S. Mao, S. O. Aderinto, X. K. Shi, K. S. Shen, and H. L. Wu, Color. Technol., 134, 230–239 (2018).
N. Singh, N. Kaur, B. McCaughan, and J. F. Callan, Tetrahedron Lett., 51, 3385–3387 (2010).
H. L. Wu, C. Y. Chen, H. Zhang, H. P. Peng, F. Wang, Z. H. Yang, and J. W. Zhang, Chem. Pap., 70, 685–694 (2016).
S. O. Aderinto, H. Zhang, H. L. Wu, C. Y. Chen, J. W. Zhang, H. P. Peng, Z. H. Yang, and F. Wang, Color. Technol., 133, 40–49 (2017).
H. L. Wu, H. P. Peng, F. Wang, H. Zhang, C. G. Chen, J. W. Zhang, and Z. H. Yang, J. Appl. Spectrosc., 83, 931–937 (2017).
X. Q. Song, Y. Q. Peng, G. Q. Cheng, X. R. Wang, P. P. Liu, and W. Y. Xu, Inorg. Chim. Acta, 427, 13–21 (2015).
J. Zhang, Y. Zhang, S. T. Zhang, X. Y. Dong, and W. K. Dong, Asian J. Chem., 27, 654–656 (2015).
W. K. Dong, Y. X. Sun, C. Y. Zhao, X. Y. Dong, and L. Xu, Polyhedron, 29, 2087–2097 (2010).
Y. J. Dong, X. Y. Dong, W. K. Dong, Y. Zhang, and L. S. Zhang, Polyhedron, 123, 305–315 (2017).
W. K. Dong, J. C. Ma, Y. J. Dong, L. Zhao, L. C. Zhu, Y. X. Sun, and Y. Zhang, J. Coord. Chem., 69, 3231–3241 (2016).
J. C. Ma, X. Y. Dong, W. K. Dong, Y. Zhang, L. C. Zhu, and J. T. Zhang. J. Coord. Chem., 69, 149–159 (2016).
W. K. Dong, P. F. Lan, W. M. Zhou, and Y. Zhang, J. Coord. Chem., 69, 1272–1283 (2016).
W. K. Dong, L. C. Zhu, J. C. Ma, Y. X. Sun, and Y. Zhang, Inorg. Chim. Acta, 53, 402–408 (2016).
Y. Gao, Y. Li, X. Yang, F. He, J. Huang, M. Jiang, Z. Zhou, and H. Chen, RSC Adv., 5, 80110–80117 (2015).
Y. Q. Xu, B. H. Li, W. W. Li, J. Zhao, S. G. Sun, and Y. Pang, Chem. Commun., 49, 4764–4766 (2013).
L. Q. Chai, K. H. Mao, J. Y. Zhang, K. Y. Zhang, and H. S. Zhang, Inorg. Chim. Acta, 457, 34–40 (2017).
N. I. Georgiev, V. B. Bojinov, and N. Marinova, Sens. Actuat. B: Chem., 150, 655–666 (2010).
X. Q. Song, P. P. Liu, Y. A. Liu, J. J. Zhou, and X. L. Wang, Dalton Trans., 45, 8154–8163 (2016).
M. H. Lim, B. A. Wong, W. H. Pitcock, Jr., D. Mokshagundam, M. H. Baik, and S. J. Lippard, J. Am. Chem. Soc., 128, 14364–14373 (2006).
N. I. Georgiev and V. B. Bojinov, J. Lumin., 132, 2235–2241 (2012).
S. Roy, P. Gayen, R. Saha, T. K. Mondal, and C. Sinha, Inorg. Chim. Acta, 410, 202–213 (2014).
N. I. Georgiev, A. M. Asiri, A. H. Qusti, K. A. Alamryb, and V. B. Bojinov, Sens. Actuat. B: Chem., 190, 185–198 (2014).
K. A. Alamry, N. I. Georgiev, S. A. EI-Daly, L. A. Taib, and V. B. Bojinov, J. Lumin., 158, 50–59 (2015).
Y. F. Liu, M. Deng, X. S. Tang, T. Zhu, Z. G. Zang, X.F. Zeng, and S. Han, Sens. Actuat. B: Chem., 233, 25–30 (2016).
W. Shen, L. Q. Yan, W. W. Tian, X. Cui, Z. J. Qi, and Y. M. Sun, J. Lumin., 177, 299–305 (2016).
J. G. Huang, M. Tang, M. Liu, M. Zhou, Z. Liu, Y. Cao, M. Y. Zhu, S. G. Liu, and W. B. Zeng, Dyes Pigments, 107, 1–8 (2014).
L. Zhao, G. Wang, J. Chen, L. Zhang, B. Liu, J. Zhang, Q. Zhao, and Y. Zhou, J. Fluorine Chem., 158, 53–59 (2014).
K. Yin, Y. X. Wu, S. S. Wang, and L. X. Chen, Sens. Actuat. B: Chem., 232, 257–263 (2016).
J. Zhang, C. W. Yu, S. Y. Qian, G. Lu, and J. L. Chen, Dyes Pigments, 92, 1370–1375 (2012).
F. P. Hou, J. Cheng, P. X. Xi, F. J. Cheng, L. Huang, G. Q. Xie, Y. J. Xie, Y. J. Shi, H. Y. Liu, D. C. Bai, and Z. Z. Zeng, Dalton Trans., 41, 5799–5804 (2012).
T. Koike, T. Watanabe, S. Aoki, E. Kimura, and M. Shiro, J. Am. Chem. Soc., 118, 12696–12703 (1996).
K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura, and T. Nagano, J. Am. Chem. Soc., 133, 18003–18005 (2011).
M. Maity, M. C. Majee, S. Kundu, S. K. Samanta, E. C. Sañudo, S. Ghosh, and M. Chaudhury, Inorg. Chem., 54, 9715−9726 (2015).
Z. Wang, Y. H. Xing, C. G. Wang, X. Q. Zeng, M. F. Ge, and S. Y. Niu, Transit. Met. Chem.,34, 655–661 (2009).
Y. Q. Sun, M. Liang, W. Dong, G. M. Yang, Dai, Z. Liao, Z. H. Jiang, S. P. Yan, and P. Cheng, Eur. J. Inorg. Chem., 7, 1514–1521 (2004).
H. Li, H. Guan, X. Duan, J. Hu, G. Wang, and Q. Wang, Org. Biomol. Chem., 11, 1805–1809 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 87, No. 3, pp. 387–394, May–June, 2020.
Rights and permissions
About this article
Cite this article
Qu, Y., Wang, C., Wu, YC. et al. Fluorescent Probe Derived from 1,8-Naphthalimide-Schiff Base for Copper(Ii) Ion: Synthesis, Characterization, and Application. J Appl Spectrosc 87, 429–436 (2020). https://doi.org/10.1007/s10812-020-01018-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-020-01018-x