Abstract
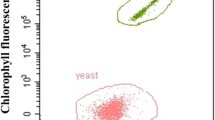
Heterotrophic or mixotrophic culture of microalgae is feasible alternative approach to avoid light limitation in autotrophic culture. However, only a few kinds of organic carbon sources are available for algal culture. Disaccharides, such as sucrose, are difficult to be utilized by microalgae under both heterotrophic and mixotrophic conditions. In this study, a symbiotic yeast was accidentally found in a contaminated algal suspension. The symbiotic yeast was isolated and identified as Cryptococcus sp. This yeast was able to extracellularly hydrolyze sucrose and accumulated monosaccharides in the medium. It can enhance algal growth using sucrose as the carbon source at both heterotrophic and mixotrophic modes when mix-cultured with Chlorella pyrenoidosa. The highest algal cell density of 118.8 × 106 and 151.2 × 106 cells/mL was achieved with a final algal percentage of 83.5 and 93.2% at heterotrophic and mixotrophic culture, respectively. Furthermore, the protein and lipid content was significantly enhanced by mix-culture C. pyrenoidosa with Cryptococcus YZU-1. The fatty acid accumulated in this co-culture system was suitable for the production of biodiesel. This symbiotic yeast solved the problem that C. pyrenoidosa cannot heterotrophically or mixotrophically utilize sucrose. A high algae density was obtained and the protein and lipid accumulation were also significantly enhanced. This study provided a novel approach for production of protein or lipid-rich biomass using sucrose or sucrose-rich wastes as the carbon source.






Similar content being viewed by others
Abbreviations
- IAA:
-
Indole-3-acetic acid
- MUFA:
-
Monounsaturated fatty acid
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
References
Chandra R, Iqbal HMN, Vishal G, Lee HS, Nagra S (2019) Algal biorefinery: a sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour Technol 278:346–359. https://doi.org/10.1016/j.biortech.2019.01.104
Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ (2018) Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol Adv 36:54–67. https://doi.org/10.1016/j.biotechadv.2017.09.009
Zhan J, Rong J, Wang Q (2017) Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int J Hydrogen Energy 42:8505–8517. https://doi.org/10.1016/j.ijhydene.2016.12.021
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36. https://doi.org/10.1016/j.watres.2010.08.037
Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771. https://doi.org/10.1002/bit.21489
Wu L, Wu S, Qiu J, Xu C, Li S, Xu H (2017) Green synthesis of isomaltulose from cane molasses by Bacillus subtilis WB800-pHA01-palI in a biologic membrane reactor. Food Chem 229:761–768. https://doi.org/10.1016/j.foodchem.2017.03.001
Zhang W, Zhang P, Sun H, Chen M, Lu S, Li P (2014) Effects of various organic carbon sources on the growth and biochemical composition of Chlorella pyrenoidosa. Bioresour Technol 173:52–58. https://doi.org/10.1016/j.biortech.2014.09.084
Kilian SG, Sutherland FCW, Meyer PS, Du Preez JC (1997) Transport-limited sucrose utilization and neokestose production by Phaffia rhodozyma. Biotechnol Lett 18:975–980
Wang SK, Wu Y, Wang X (2016) Heterotrophic cultivation of Chlorella pyrenoidosa using sucrose as the sole carbon source by co-culture with Rhodotorula glutinis. Bioresour Technol 220:615–620. https://doi.org/10.1016/j.biortech.2016.09.010
Wang SK, Wang X, Tao HH, Sun XS, Tian YT (2018) Heterotrophic culture of Chlorella pyrenoidosa using sucrose as the sole carbon source by co-culture with immobilized yeast. Bioresour Technol 249:425–430. https://doi.org/10.1016/j.biortech.2017.10.049
Dao GH, Wu GX, Wang XX, Zhang TY, Zhan XM, Hu HY (2018) Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res 33:345–351. https://doi.org/10.1016/j.algal.2018.06.006
Xue LG, Shang H, Ma P, Wang X, He XY, Niu JB, Wu JL (2018) Analysis of growth and lipid production characteristics of Chlorella vulgaris in artificially constructed consortia with symbiotic bacteria. J Basic Microbiol 58:358–367. https://doi.org/10.1002/jobm.201700594
Amor DR, Bello MD (2019) Bottom-up approaches to synthetic cooperation in microbial communities. Life 9:22. https://doi.org/10.3390/life9010022
Smith RT, Bangert K, Wilkinson SJ, Gilmour DJ (2015) Synergistic carbon metabolism in a fast growing mixotrophic freshwater microalgal species Micractinium inermum. Biomass Bioenergy 82:73–86. https://doi.org/10.1016/j.biombioe.2015.04.023
Foster RA, Kuypers MMM, Vagner T, Paerl RW, Musat N, Zehr JP (2011) Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses. ISME J 5:1484–1493. https://doi.org/10.1038/ismej.2011.26
Haines KC, Guillard RRL (1974) Growth of vitamin B12-requiring marine diatoms in mixed laboratory cultures with vitamin B12-producing marine bacteria. J Phycol 10:245–252. https://doi.org/10.1111/j.1529-8817.1974.tb02709.x
Tandon P, Jin Q, Huang L (2017) A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb Cell Fact 16:219. https://doi.org/10.1186/s12934-017-0834-2
Zhou W, Cheng Y, Li Y, Wan Y, Liu Y, Lin X, Ruan R (2012) Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl Biochem Biotechnol 167:214–228. https://doi.org/10.1007/s12010-012-9667-y
Shen P, Chen X (2006) Microbiology, 2nd edn. Higher Education Press, Beijing
Fontana A, Ghommidh C, Guiraud JP, Navarro JM (1992) Continuous alcoholic fermentation of sucrose using flocculating yeast. The limits of invertase activity. Biotechnol Lett 14:505–510. https://doi.org/10.1007/BF01023176
Costaglioli P, Meilhoc F, Janatova I, Klein R, Masson J (1997) Secretion of invertase from Schwanniomyces occidentalis. Biotechnol Lett 19:623–627. https://doi.org/10.1023/A:1018326512461
Francisco EC, Franco TT, Wagner R, Jacob-Lopes E (2014) Assessment of different carbohydrates as exogenous carbon source in cultivation of cyanobacteria. Bioprocess Biosyst Eng 37:1497–1505. https://doi.org/10.1007/s00449-013-1121-1
Sun N, Wang Y, Li YT, Huang JC, Chen F (2008) Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochem 43:1288–1292. https://doi.org/10.1016/j.procbio.2008.07.014
Zhang HF, Wang WL, Li YG, Yang WJ, Shen GM (2011) Mixotrophic cultivation of Botryococcus braunii. Biomass Bioenerg 35:1710–1715. https://doi.org/10.1016/j.biombioe.2011.01.002
Lin TS, Wu JY (2015) Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour Technol 184:100–107. https://doi.org/10.1016/j.biortech.2014.11.005
Reid SJ, Abratt VR (2005) Sucrose utilisation in bacteria: genetic organization and regulation. Appl Microbiol Biotechnol 67:312–321. https://doi.org/10.1007/s00253-004-1885-y
Seiboth B, Pakdaman BS, Hartl L, Kubicek CP (2007) Lactose metabolism in filamentous fungi: how to deal with an unknown substrate. Fungal Biol Rev 1:42–48. https://doi.org/10.1016/j.fbr.2007.02.006
Rajapitamahuni S, Bachani P, Sardar RK, Mishra S (2019) Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J Appl Phycol 31:29–39. https://doi.org/10.1007/s10811-018-1591-2
Li H, Zhong Y, Lu Q, Zhang X, Wang Q, Liu H, Diao Z, Yao C, Liu H (2019) Co-cultivation of Rhodotorula glutinis and Chlorella pyrenoidosa to improve nutrient removal and protein content by their synergistic relationship. RSC Adv 9:14331–14342. https://doi.org/10.1039/C9RA01884K
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160:498–503. https://doi.org/10.1007/s12010-008-8376-z
Zhang Z, Ji H, Gong G, Zhang X, Tan T (2014) Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour Technol 164:93–99. https://doi.org/10.1016/j.biortech.2014.04.039
Yen HW, Chen PW, Chen LJ (2015) The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol 184:148–152. https://doi.org/10.1016/j.biortech.2014.09.113
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507. https://doi.org/10.1016/j.jbiotec.2006.05.002
Deshmukh S, Kumar R, Bala K (2019) Microalgae biodiesel: a review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process Technol 191:232–247. https://doi.org/10.1016/j.fuproc.2019.03.013
Wang SK, Wang X, Miao J, Tian YT (2018) Tofu whey wastewater is a promising basal medium for microalgae culture. Bioresour Technol 253:79–84. https://doi.org/10.1016/j.biortech.2018.01.012
Zhang Z, Sun D, Wu T, Li Y, Lee Y, Liu J, Chen F (2017) The synergistic energy and carbon metabolism under mixotrophic cultivation reveals the coordination between photosynthesis and aerobic respiration in Chlorella zofingiensis. Algal Res 25:109–116. https://doi.org/10.1016/j.algal.2017.05.007
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 31802323), Natural Science Foundation of Jiangsu Province, China (no. BK20170495), and High-level Talent Support Program of Yangzhou University.
Author information
Authors and Affiliations
Contributions
YT and XW carried out the experiments, analyzed the data and drafted the manuscript. YC contributed analytic methods. SW designed the experiments, analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, YT., Wang, X., Cui, YH. et al. A symbiotic yeast to enhance heterotrophic and mixotrophic cultivation of Chlorella pyrenoidosa using sucrose as the carbon source. Bioprocess Biosyst Eng 43, 2243–2252 (2020). https://doi.org/10.1007/s00449-020-02409-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02409-2