Abstract
The use of agro-industrial by-products as feedstock for selenium-enriched yeast biomass production is an innovative and low-cost method. Thus, the present study aims for the utilization of sugarcane bagasse and corn bran hydrolysates for the production of selenium-enriched yeast and the evaluation of the effect of selenium in growth and cell composition. The hydrolysates were obtained using acid pretreatment, and yeasts were evaluated in different medium compositions. All evaluated yeasts were able to grow in the presence of 15 mg/L selenium. S. cerevisiae strains presented the major tolerance to selenium and better relation between selenium uptake and cell growth, with maximum cell biomass production of 7.97 ± 0.24 g/L and maximum selenium uptake of 1193 ± 336 ppm and 99% cell viability. Selenium also enhanced the lipid and protein concentration in cell extracts obtained after mechanical disruption. Regarding agro-industrial hydrolysates, corn bran hydrolysate allowed a better growth, with the production of 4.25 ± 0.49 g/L of biomass enriched with 167 ± 18 ppm selenium and 100% of viability. Starchy and lignocellulosic biomasses presented potential as low-cost alternatives for single-cell protein contributing to the development of sustainable and economically viable technologies.

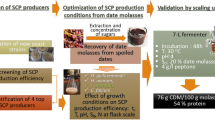
Graphical abstract



Similar content being viewed by others
References
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812. https://doi.org/10.1016/j.biopha.2018.12.146
Kieliszek M, Kot AM, Bzducha-Wróbel A, Błazejak S, Gientka I, Kurcz A (2017) Biotechnological use of Candida yeasts in the food industry: a review. Fungal Biol Rev 31:185–198. https://doi.org/10.1016/j.fbr.2017.06.001
Maiyo F, Singh M (2017) Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine 12. https://doi.org/10.2217/nnm-2017-0024
Paiva FA, Netto AS, Corrêa LB, Silva TH, Guimarães ICSB, Del Claro GR, Cunha JA, Zanetti MA (2019) Organic selenium supplementation increases muscle selenium content in growing lambs compared to inorganic source. Small Rumin Res 175:57–64. https://doi.org/10.1016/j.smallrumres.2019.04.008
Papadomichelakis G, Zoidis E, Pappas AC, Danezis G, Georgiou CA, Fegeros K (2018) Dietary organic selenium addition and accumulation of toxic and essential trace elements in liver and meat of growing rabbits. Meat Sci 145:383–388. https://doi.org/10.1016/j.meatsci.2018.07.022
Kieliszek M, Błażejak S, Kurek E (2017) Binding and conversion of selenium in Candida utilis ATCC 9950 yeasts in bioreactor culture. Molecules 22:352. https://doi.org/10.3390/molecules22030352
Kieliszek M, Błażejak S, Gientka I, Bzducha-Wróbel A (2015) Accumulation and metabolism of selenium by yeast cells. Appl Microbiol Biotechnol 99:5373–5382. https://doi.org/10.1007/s00253-015-6650-x
Shurson GC (2018) Yeast and yeast derivatives in feed additives and ingredients: sources, characteristics, animal responses, and quantification methods. Anim Feed Sci Technol 235:60–76. https://doi.org/10.1016/j.anifeedsci.2017.11.010
P&S Intelligence (2018). Protein extracts from single cell protein sources Market Overview. https://www.psmarketresearch.com/market-analysis/protein-extracts-from-single-cell-protein-sources-market,
Juszczyk P, Rymowicz W, Kita A, Rywińska A (2019) Biomass production by Yarrowia lipolytica yeast using waste derived from the production of ethyl esters of polyunsaturated fatty acids of flaxseed oil. Ind Crop Prod 138:111590. https://doi.org/10.1016/j.indcrop.2019.111590
Andrietta MGS, Steckelberg C, Kitaka PR, Goldebeck R, Andrietta SR (2017) Yeast from ethanol production–source of scp (single cell protein). WJRR 4:87–89
Nurcholis M, Lertwattanasakul N, Rodrussamee N, Kosaka T, Murata M, Yamada M (2020) Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl Microbiol Biotechnol 104:475–488. https://doi.org/10.1007/s00253-019-10224-3
Santos JF, Canettieri EV, Souza SMA, Rodrigues RCLB, Martínez EA (2019) Treatment of sugarcane vinasse from cachaça production for the obtainment of Candida utilis CCT 3469 biomass. Biochem Eng J 148:131–137. https://doi.org/10.1016/j.bej.2019.04.009
Morin N, Czerwiec Q, Nicaud J-M, Neuvéglise C, Rossignol T (2020) Transforming Candida hispaniensis, a promising oleaginous and flavogenic yeast. Yeast 37:348–355. https://doi.org/10.1002/yea.3466
Louhasakul Y, Cheirsilp B, Maneerat S, Prasertsan P (2019) Potential use of flocculating oleaginous yeasts for bioconversion of industrial wastes into biodiesel feedstocks. Renew Energy 136:1311–1319
Egressy-Molnár O, Ouerdane L, Győrfi J, Dernovics M (2016) Analogy in selenium enrichment and selenium speciation between selenized yeast Saccharomyces cerevisiae and Hericium erinaceus (lion’s mane mushroom). LWT-Food Sci Technol 68:306–312. https://doi.org/10.1016/j.lwt.2015.12.028
Vallejos ME, Kruyeniski J, Area MC (2017) Second-generation bioethanol from industrial wood waste of South American species. Biofuel Res J 15:654–667
Lee JE, Vadlani PV, Faubion J (2017) Corn bran bioprocessing: development of an integrated process for microbial lipids production. Bioresour Technol 243:196–203. https://doi.org/10.1016/j.biortech.2017.06.065
Wobiwo FA, Chaturvedi T, Boda M, Fokou E, Emaga TH, Cybulska I, Deleu M, Gerin PA, Thomsen MH (2019) Bioethanol potential of raw and hydrothermally pretreated banana bulbs biomass in simultaneous saccharification and fermentation process with Saccharomyces cerevisiae. Biomass Convers Biorefin 9:553–563. https://doi.org/10.1007/s13399-018-00367-0
Juszczyk P, Rymowicz W, Kita A, Rywińska A (2019) Biomass production by Yarrowia lipolytica yeast using waste derived from the production of ethyl sters of polyunsaturated fatty acids of flaxseed oil. Ind Crop Prod 138:111590. https://doi.org/10.1016/j.indcrop.2019.111590
Silva DDV, Dussán KM, Hernández V, Silva SS, Cardona CA, Felipe MCA (2016) Effect of volumetric oxygen transfer coefficient (kLa) on ethanol production performance by Scheffersomyces stipitis on hemicellulosic sugarcane bagasse hydrolysate. Biochem Eng J 112:249–257. https://doi.org/10.1016/j.bej.2016.04.012
Martiniano, S. E.: Produção de leveduras enriquecidas com selênio a partir de resíduos vegetais. PhD thesis, University of São Paulo, Lorena (2017)
Martiniano SE, Philippini RR, Chandel AK, Rosa CA, Pagnocca FC, da Silva SS (2014) Evaluation of rice bran extract as a nitrogen source for improved hemicellulosic ethanol production from sugarcane bagasse by new xylose-fermenting yeast strains isolated from brazilian forests. Sugar Tech 16:1–8. https://doi.org/10.1007/s12355-013-0219-8
Oliveira CGR (2006) Desenvolvimento de bioprocesso para a produção de biomassa de levedura (Saccharomyces cerevisiae) rica em organo-selênio. Universidade Federal do Paraná, Curitiba, Dissertation
Chávez JDR (2008) Aproveitamento biotecnólogico do glicerol derivado da produção de biodiesel para a obtenção de biomassa e ribunocleotídeos. University of São Paulo, Lorena, Dissertation
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Izard J, Limberger RJ (2003) Rapid screening method for quantitation of bacterial cell lipids from whole cells. J Microbiol Methods 55:411–418
Narayana B, Mathew M, Bhat NG, Sreekumar NV (2003) Spectrophotometric determination of selenium using potassium iodide and starch as reagents. Microchim Acta 141:175–178
Martins DSR, Leocádio LG, Da Silveria CLP (2008) ICP-OES simultaneous determination of Ca, Cu, Fe, Mg, Mn, Na and P in biodiesel by axial and radial inductively coupled plasma-optical emission spectrometry. Anal Lett 41:1615–1622
Ellis D, Davis S, Alexiou H, Handke R, Bartley R (2007) Descriptions of medical fungi. Nexus Print Solutions, Adelaide
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kot AM (2019) Effect of selenium on growth and antioxidative system of yeast cells. Mol Biol Rep 46:1797–1808. https://doi.org/10.1007/s11033-019-04630-z
Ceccato-Antonini SR, Sudbery PE (2004) Filamentous growth in Saccharomyces cerevisiae. Braz J Microbiol 35:173–181
Kieliszek M, Błażejak S, Płaczek M (2016) Spectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeast. J Trace Elem Med Biol 35:90–96. https://doi.org/10.1016/j.jtemb.2016.01.014
Čertík M, Breierová E, Oláhová M, Šajbidor J, Márová I (2013) Effect of selenium on lipid alternations in pigment-forming yeasts. Food Sci Biotechnol 22:45–51
Zhang G, Yao X, Wang C, Wang D, Wei G (2019) Transcriptome analysis reveals the mechanism underlying improved glutathione biosynthesis and secretion in Candida utilis during selenium enrichment. J Biotechnol 304:89–96. https://doi.org/10.1016/j.jbiotec.2019.08.015
Hamza F, Vaidya A, Apte M, Kumar AR, Zinjarde S (2017) Selenium nanoparticle-enriched biomass of Yarrowia lipolytica enhances growth and survival of Artemia salina. Enzym Microb Technol 106:48–54. https://doi.org/10.1016/j.enzmictec.2017.07.002
Yang B, Wang D, Wei G, Liu Z, Ge X (2013) Selenium-enriched Candida utilis: efficient preparation with l-methionine and antioxidant capacity in rats. J Trace Elem Med Biol 27:7–11
Dijken JP, Scheffers WA (1986) Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev 32:199–224
Philippini RR, Martiniano SE, Chandel AK, Carvalho W, da Silva SS (2017) Pretreatment of sugarcane bagasse from cane hybrids: effects on chemical composition and 2G sugars recovery. Waste Biomass Valori 10:1561–1570. https://doi.org/10.1007/s12649-017-0162-0
Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101:4775–4800
Olsson L, Hahn-Hägerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Tech 18:312–331
Nanis I, Hatzikamari M, Katharopoulos E, Boukouvala E, Ekateriniadou L, Litopoulou-Tzanetaki E, Gerasopoulos D (2020) Microbiological and physicochemical changes during fermentation of solid residue of olive mill wastewaters: exploitation towards the production of an olive paste–type product. LWT-Food Sci Technol 117:108671. https://doi.org/10.1016/j.lwt.2019.108671
Solovyev N, Prakash NT, Bhatia P, Prakash R, Drobyshev E, Michalke B (2018) Selenium-rich mushrooms cultivation on a wheat straw substrate from seleniferous area in Punjab India. J Trace Elem Med Bio 50:362–366. https://doi.org/10.1016/j.jtemb.2018.07.027
Acknowledgments
The authors would like to thank Professor Carlos Augusto Rosa (UFMG, Brazil) and Professor Fernando Carlos Pagnocca (UNESP, Brazil) for the yeast strains.
Funding
This study received financial support provided by the Research Council for the State of São Paulo (Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP—Process No. 2016/10636-8), the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—USP-CAPES program), and the Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of novelty
Selenium is a trace element essential for both human and animal health, preventing and combating several diseases, and these benefits are increased when selenium is incorporated by yeasts. The use of agro-industrial by-products as feedstocks assists in the development of a more cost-benefit and eco-friendly biomolecules. The present study is the first report of selenium-enriched yeast production using agro-industrial by-products as both carbon and nitrogen sources. We also evaluated the effects of selenium uptake in different yeasts, generally used in animal feed. Sugarcane bagasse and corn bran hydrolysates as fermentative media enabled the production of yeast biomass enriched with selenium, presenting potential as low-cost alternatives for mineral-enriched single-cell protein and contributing to the development of sustainable and economically viable technologies.
Rights and permissions
About this article
Cite this article
Martiniano, S.E., Philippini, R.R., Franco-Marcelino, P.R. et al. Effect of selenium uptake on growth metabolism in yeasts for the production of enriched single-cell protein using agro-industrial by-products. Biomass Conv. Bioref. 12, 3975–3983 (2022). https://doi.org/10.1007/s13399-020-00885-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00885-w