The Use of Factorial Design and Simplex Optimization to Improve Analytical Performance of In Situ Film Electrodes
Abstract
:1. Introduction
2. Experimental
2.1. Solution Preparation
2.2. Electrochemical Measurements
2.3. Evaluation of the Performance of the SWASV Pethod
3. Results and Discussion
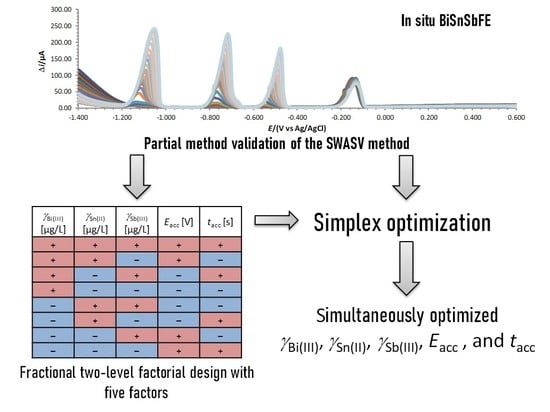
3.1. Fractional Factorial Design
3.2. Simplex Optimization
3.3. The Linearity, Sensitivity, LOD, LOQ, and Precision of the System for Different In Situ FEs
3.4. Comparison of the Optimized In Situ FE with the Pure In Situ FEs
3.5. Interference Study
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Švancara, I.; Prior, C.; Hočevar, S.B.; Wang, J. A Decade with Bismuth-Based Electrodes in Electroanalysis. Electroanalysis 2010, 22, 1405–1420. [Google Scholar] [CrossRef]
- Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Antimony-Based electrodes for analytical determinations. TrAC Trends Anal. Chem. 2016, 77, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Pauliukaite, R.; Metelka, R.; Švancara, I.; Królicka, A.; Bobrowski, A.; Norkus, E.; Kalcher, K.; Vytřas, K. Screen-Printed carbon electrodes bulk-modified with Bi2O3 OR Sb2O3 for trace determination of some heavy metals. Sci. Pap. Univ. Pardubic. Ser. A 2004, 10, 47–58. [Google Scholar]
- Shehata, M.; Fekry, A.M.; Walcarius, A. Moxifloxacin Hydrochloride Electrochemical Detection at Gold Nanoparticles Modified Screen-Printed Electrode. Sensors 2020, 20, 2797. [Google Scholar] [CrossRef]
- Feng, X.-Z.; Ferranco, A.; Su, X.; Chen, Z.; Jiang, Z.; Han, G.-C. A Facile Electrochemical Sensor Labeled by Ferrocenoyl Cysteine Conjugate for the Detection of Nitrite in Pickle Juice. Sensors 2019, 19, 268. [Google Scholar] [CrossRef] [Green Version]
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [Green Version]
- Jovanovski, V.; Hočevar, S.B.; Ogorevc, B. Bismuth electrodes in contemporary electroanalysis. Curr. Opin. Electrochem. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Czop, E.; Economou, A.; Bobrowski, A. A study of in situ plated tin-film electrodes for the determination of trace metals by means of square-wave anodic stripping voltammetry. Electrochim. Acta 2011, 56, 2206–2212. [Google Scholar] [CrossRef]
- Yuan, H.; Li, N.; Linghu, J.; Dong, J.; Wang, Y.; Karmakar, A.; Yuan, J.; Li, M.; Buenconsejo, P.J.S.; Liu, G.; et al. Chip-Level Integration of Covalent Organic Frameworks for Trace Benzene Sensing. ACS Sens. 2020, 5, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Colozza, N.; Cacciotti, I.; Moscone, D.; Arduini, F. Effects of Humidity, Temperature and Bismuth Electrodeposition on Electroanalytical Performances of Nafion-Coated Printed Electrodes for Cd2+ and Pb2+ Detection. Electroanalysis 2020, 32, 345–357. [Google Scholar] [CrossRef]
- Hou, X.; Xiong, B.; Wang, Y.; Wang, L.; Wang, H. Determination of trace lead and cadmium in decorative material using disposable screen-printed electrode electrically modified with reduced graphene oxide/L-cysteine/Bi-film. Sensors 2020, 20, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katseli, V.; Thomaidis, N.; Economou, A.; Kokkinos, C. Miniature 3D-Printed integrated electrochemical cell for trace voltammetric Hg(II) determination. Sens. Actuators B Chem. 2020, 308, 127715. [Google Scholar] [CrossRef]
- Kava, A.A.; Beardsley, C.; Hofstetter, J.; Henry, C.S. Disposable glassy carbon stencil printed electrodes for trace detection of cadmium and lead. Anal. Chim. Acta 2020, 1103, 58–66. [Google Scholar] [CrossRef]
- Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-Wrapped carbon nanotube composite films coated on diazonium-modified flexible ITO sheets for the electroanalysis of heavy metal ions. Sensors 2020, 20, 580. [Google Scholar] [CrossRef] [Green Version]
- Dang, X.; Zhang, X.; Zhao, H. Signal amplified photoelectrochemical sensing platform with g-C3N4/inverse opal photonic crystal WO3 heterojunction electrode. J. Electroanal. Chem. 2019, 840, 101–108. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Schirhagl, R.; Quan, X. Two-Dimensional nanomaterial based sensors for heavy metal ions. Microchim. Acta 2018, 185, 478. [Google Scholar] [CrossRef]
- Du, J.; Fan, Y.; Gan, X.; Dang, X.; Zhao, H. Three-Dimension branched crystalline carbon nitride: A high efficiency photoelectrochemical sensor of trace Cu2+ detection. Electrochim. Acta 2020, 330, 135336. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Jain, R.; Zhao, H.; Karolia, P.; Jadon, N. Voltammetric sensing based on the use of advanced carbonaceous nanomaterials: A review. Microchim. Acta 2018, 185, 89. [Google Scholar] [CrossRef]
- Rong, R.; Zhao, H.; Gan, X.; Chen, S.; Quan, X. An electrochemical sensor based on graphene-polypyrrole nanocomposite for the specific detection of Pb (II). Nano 2017, 12, 1750008. [Google Scholar] [CrossRef]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-Based Electrochemical Potentiostat Detection System Using PEDOT: PSS/Chitosan/Graphene Modified Screen-Printed Electrodes for Dopamine Detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef]
- Finšgar, M.; Kovačec, L. Copper-Bismuth-Film in situ electrodes for heavy metal detection. Microchem. J. 2020, 154, 104635. [Google Scholar] [CrossRef]
- Finšgar, M.; Petovar, B. Novel in situ Bi−Sb-Film Electrodes for Trace Heavy Metal Analysis. Electroanalysis 2018, 30, 2781–2792. [Google Scholar] [CrossRef]
- Finšgar, M.; Petovar, B.; Vodopivec, K. Bismuth-Tin-Film electrodes for Zn(II), Cd(II), and Pb(II) trace analysis. Microchem. J. 2019, 145, 676–685. [Google Scholar] [CrossRef]
- Finšgar, M.; Xhanari, K.; Petovar, B. A Comparison of Hydrochloric Acid and Acetate Buffer Media for Trace Metal Analysis Using Sb-Film Electrodes: A Detailed Electrochemical Impedance Spectroscopy Study. J. Electrochem. Soc. 2019, 166, H108–H118. [Google Scholar] [CrossRef]
- Finšgar, M.; Xhanari, K.; Petovar, B. Copper-film electrodes for Pb(II) trace analysis and a detailed electrochemical impedance spectroscopy study. Microchem. J. 2019, 147, 863–871. [Google Scholar] [CrossRef]
- Dabrowska, S.; Migdalski, J.; Lewenstam, A. Direct Potentiometric Determination of Hydrogen Carbonate in Mineral Waters. Electroanalysis 2017, 29, 140–145. [Google Scholar] [CrossRef]
- Bia, G.; Borgnino, L.; Ortiz, P.I.; Pfaffen, V. Multivariate optimization of square wave voltammetry using bismuth film electrode to determine atrazine. Sens. Actuators B Chem. 2014, 203, 396–405. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.; Jong, S.D.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part. A; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Kefala, G.; Economou, A.; Voulgaropoulos, A.; Sofoniou, M. A study of bismuth-film electrodes for the detection of trace metals by anodic stripping voltammetry and their application to the determination of Pb and Zn in tapwater and human hair. Talanta 2003, 61, 603–610. [Google Scholar] [CrossRef]
- Lin, L.; Lawrence, N.S.; Thongngamdee, S.; Wang, J.; Lin, Y. Catalytic adsorptive stripping determination of trace chromium (VI) at the bismuth film electrode. Talanta 2005, 65, 144–148. [Google Scholar] [CrossRef]
- Tesarova, E.; Baldrianova, L.; Hocevar, S.B.; Svancara, I.; Vytras, K.; Ogorevc, B. Anodic stripping voltammetric measurement of trace heavy metals at antimony film carbon paste electrode. Electrochim. Acta 2009, 54, 1506–1510. [Google Scholar] [CrossRef]
- Bassie, T.; Siraj, K.; Tesema, T.E. Determination of Heavy Metal Ions on Glassy Carbon Electrode Modified with Antimony. Adv. Sci. Eng. Med. 2013, 5, 275–284. [Google Scholar] [CrossRef]
- Hocevar, S.B.; Švancara, I.; Ogorevc, B.; Vytřas, K. Antimony Film Electrode for Electrochemical Stripping Analysis. Anal. Chem. 2007, 79, 8639–8643. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; dos Santos, Q.O.; Santos, A.G.; Novaes, C.G.; Ferreira, S.L.C.; de Souza, V.S. Simplex optimization: A tutorial approach and recent applications in analytical chemistry. Microchem. J. 2016, 124, 45–54. [Google Scholar] [CrossRef]
- Łukasz, K.; Yvan, V.H.; Sherma, J. Chemometrics in Chromatography, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Anal. Chim. Acta 2016, 916, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutton, L.A.; Newton, M.E.; Unwin, P.R.; Macpherson, J.V. Factors Controlling Stripping Voltammetry of Lead at Polycrystalline Boron Doped Diamond Electrodes: New Insights from High-Resolution Microscopy. Anal. Chem. 2011, 83, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Finšgar, M.; Majer, D.; Maver, U.; Maver, T. Reusability of SPE and Sb-Modified SPE Sensors for Trace Pb(II) Determination. Sensors 2018, 18, 3976. [Google Scholar] [CrossRef] [Green Version]
- Laboratory and Scientific Section United Nations Office on Drugs and Crime. Guidance for the Validation of Analytical Methodology and Calibration of Equipment Used for Testing of Illicit Drugs in Seized Materials and Biological Specimens, A commitment to Quality and Continuous Improvement; United Nations: New York, NY, USA, 2009. [Google Scholar]
- Jovanovski, V.; Hrastnik, N.I. Insights into the anodic stripping voltammetric behaviour of copper film electrodes for determination of trace mercury. Microchem. J. 2019, 146, 895–899. [Google Scholar] [CrossRef]
- Zidarič, T.; Jovanovski, V.; Menart, E.; Zorko, M.; Kolar, M.; Veber, M.; Hočevar, S.B. Multi-Pulse galvanostatic preparation of nanostructured bismuth film electrode for trace metal detection. Sens. Actuators B Chem. 2017, 245, 720–725. [Google Scholar] [CrossRef]
- Jovanovski, V.; Hrastnik, N.I.; Hočevar, S.B. Copper film electrode for anodic stripping voltammetric determination of trace mercury and lead. Electrochem. Commun. 2015, 57, 1–4. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Bi, Y.; Ma, C.; Bai, J.; Hu, Z.; Zhou, M. Biomass derived worm-like nitrogen-doped-carbon framework for trace determination of toxic heavy metal lead (II). Anal. Chim. Acta 2020, 1116, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calvo, A.; Blanco-López, M.C.; Costa-García, A. Paper-Based Working Electrodes Coated with Mercury or Bismuth Films for Heavy Metals Determination. Biosensors 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, G. Synthesis of a three-dimensional (BiO)2CO3@single-walled carbon nanotube nanocomposite and its application for ultrasensitive detection of trace Pb(II) and Cd(II) by incorporating Nafion. Sens. Actuators B Chem. 2019, 288, 71–79. [Google Scholar] [CrossRef]
- Bedin, K.C.; Mitsuyasu, E.Y.; Ronix, A.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. Inexpensive bismuth-film electrode supported on pencil-lead graphite for determination of Pb(II) and Cd(II) Ions by anodic stripping voltammetry. Int. J. Anal. Chem. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marija, S.; Hocevar, S.B.; Lucie, B.; Eva, T.; Ivan, S.; Bozidar, O.; Karel, V. Antimony Film Microelectrode for Anodic Stripping Measurement of Cadmium(II), Lead(II) and Copper(II). Electroanalysis 2010, 22, 1617–1622. [Google Scholar]
- Tian, Y.Q.; Li, N.B.; Luo, H.Q. Simultaneous Determination of Trace Zinc(II) and Cadmium(II) by Differential Pulse Anodic Stripping Voltammetry Using a MWCNTs–NaDBS Modified Stannum Film Electrode. Electroanalysis 2009, 21, 2584–2589. [Google Scholar] [CrossRef]
- Ariño, C.; Serrano, N.; Díaz-Cruz, J.M.; Esteban, M. Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal. Chim. Acta 2017, 990 (Suppl. C), 11–53. [Google Scholar] [CrossRef] [Green Version]
- Maczuga, M.; Economou, A.; Bobrowski, A.; Prodromidis, M.I. Novel screen-printed antimony and tin voltammetric sensors for anodic stripping detection of Pb(II) and Cd(II). Electrochim. Acta 2013, 114, 758–765. [Google Scholar] [CrossRef]
- Jovanovski, V.; Hocevar, S.B.; Ogorevc, B. Ex Situ Prepared Antimony Film Electrode for Electrochemical Stripping Measurement of Heavy Metal Ions. Electroanalysis 2009, 21, 2321–2324. [Google Scholar] [CrossRef]
- Sosa, V.; Barceló, C.; Serrano, N.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Antimony film screen-printed carbon electrode for stripping analysis of Cd(II), Pb(II), and Cu(II) in natural samples. Anal. Chim. Acta 2015, 855, 34–40. [Google Scholar] [CrossRef]
- Sebez, B.; Ogorevc, B.; Hocevar, S.B.; Veber, M. Functioning of antimony film electrode in acid media under cyclic and anodic stripping voltammetry conditions. Anal. Chim. Acta 2013, 785, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, V.; Vytřas, K.; Kuhn, A. Macroporous antimony film electrodes for stripping analysis of trace heavy metals. Electrochem. Commun. 2010, 12, 114–117. [Google Scholar] [CrossRef]
- Lezi, N.; Economou, A.; Dimovasilis, P.A.; Trikalitis, P.N.; Prodromidis, M.I. Disposable screen-printed sensors modified with bismuth precursor compounds for the rapid voltammetric screening of trace Pb(II) and Cd(II). Anal. Chim. Acta 2012, 728, 1–8. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Raptis, I.; Efstathiou, C.E. Lithographically fabricated disposable bismuth-film electrodes for the trace determination of Pb(II) and Cd(II) by anodic stripping voltammetry. Electrochim. Acta 2008, 53, 5294–5299. [Google Scholar] [CrossRef]
- Hwang, G.-H.; Han, W.-K.; Park, J.-S.; Kang, S.-G. An electrochemical sensor based on the reduction of screen-printed bismuth oxide for the determination of trace lead and cadmium. Sens. Actuators B Chem. 2008, 135, 309–316. [Google Scholar] [CrossRef]
- Kadara, R.O.; Tothill, I.E. Development of disposable bulk-modified screen-printed electrode based on bismuth oxide for stripping chronopotentiometric analysis of lead (II) and cadmium (II) in soil and water samples. Anal. Chim. Acta 2008, 623, 76–81. [Google Scholar] [CrossRef] [PubMed]







| Experiment No. | x1 | x2 | x3 | x4 | x5 | γBi(III) [µg/L] | γSn(II) [µg/L] | γSb(III) [µg/L] | Eacc [V] | tacc [s] | OCcombined | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | 0.80 | 0.70 | 0.80 | −1.500 | 90 | 0.002056 | |
| 2 | + | + | − | + | − | 0.60 | 0.80 | 0.30 | −1.500 | 30 | 0.000142 | |
| 3 | + | − | + | − | + | 0.70 | 0.20 | 0.70 | −1.300 | 80 | 0.000056 | |
| 4 | + | − | − | − | − | 0.80 | 0.30 | 0.20 | −1.300 | 20 | 0.000243 | |
| 5 | − | + | + | − | − | 0.20 | 0.80 | 0.80 | −1.200 | 40 | 0.000015 | |
| 6 | − | + | − | − | + | 0.20 | 0.70 | 0.30 | −1.200 | 70 | 0.000008 | |
| 7 | − | − | + | + | − | 0.30 | 0.30 | 0.60 | −1.400 | 30 | 0.000026 | |
| 8 | − | − | − | + | + | 0.20 | 0.20 | 0.20 | −1.400 | 60 | 0.000094 | |
| Decision level | 0.50 | 0.50 | 0.50 | −1.350 | 55 | |||||||
| Factor impact | 0.000588 | 0.000450 | 0.000417 | 0.000499 | 0.000447 | |||||||
| Critical value | 0.001172 | 0.001232 | 0.001244 | 0.001213 | 0.001233 | |||||||
| Significant impact? | No | No | No | No | No | |||||||
| Response | γBi(III) | γSn(II) | γSb(III) | Eacc | tacc |
|---|---|---|---|---|---|
| response as a combination of the product | |||||
| OCLOD = linearityZn(II) · linearityCd(II) · linearityPb(II) | No | No | No | No | No |
| OCLOD = LODZn(II) · LODCd(II) · LODPb(II) | No | No | No | No | No |
| OCLOQ = LOQZn(II) · LOQCd(II) · LOQPb(II) | No | No | No | No | Yes |
| OCslope = slopeZn(II) · slopeCd(II) · slopePb(II) | No | No | No | No | No |
| OCRSD = RSDZn(II) · RSDCd(II) · RSDPb(II) | No | No | No | No | No |
| OCRe = |100–ReZn(II)| · |100–ReCd(II)| · |100–RePb(II)| | No | No | No | No | No |
| response as a combination of the sum | |||||
| OCLOD = linearityZn(II) + linearityCd(II) + linearityPb(II) | No | No | No | No | Yes |
| OCLOD = LODZn(II) + LODCd(II) + LODPb(II) | No | No | No | No | No |
| OCLOQ = LOQZn(II) + LOQCd(II) + LOQPb(II) | No | No | No | No | Yes |
| OCslope = slopeZn(II) + slopeCd(II) + slopePb(II) | No | No | No | No | Yes |
| OCRSD = RSDZn(II) + RSDCd(II) + RSDPb(II) | No | No | No | No | Yes |
| OCRe = |100–ReZn(II)| + |100–ReCd(II)| + |100–RePb(II)| | No | No | No | No | No |
| Experiment No. | γBi(III) [mg/L] | γSn(II) [mg/L] | γSb(III) [mg/L] | Eacc [V] | tacc [s] | OCcombined | |
|---|---|---|---|---|---|---|---|
| Factorial Design | |||||||
| 1 | 0.80 (3.8 µmol/L) | 0.70 (5.9 µmol/L) | 0.80 (6.6 µmol/L) | −1.500 | 90 | 0.002056 | W7 |
| 4 | 0.80 (3.8 µmol/L) | 0.30 (2.5 µmol/L) | 0.20 (1.6 µmol/L) | −1.300 | 20 | 0.000243 | W5 |
| 2 | 0.60 (2.9 µmol/L) | 0.80 (6.7 µmol/L) | 0.30 (2.5 µmol/L) | −1.500 | 30 | 0.000142 | W4 |
| 8 | 0.20 (1.0 µmol/L) | 0.20 (1.7 µmol/L) | 0.20 (1.6 µmol/L) | −1.400 | 60 | 0.000094 | W3 |
| 3 | 0.70 (3.3 µmol/L) | 0.20 (1.7 µmol/L) | 0.70 (5.7 µmol/L) | −1.300 | 80 | 0.000056 | W2 |
| 7 | 0.30 (1.4 µmol/L) | 0.30 (2.5 µmol/L) | 0.60 (4.9 µmol/L) | −1.400 | 30 | 0.000026 | W1 |
| 5 | 0.20 (1.0 µmol/L) | 0.80 (6.7 µmol/L) | 0.80 (6.6 µmol/L) | −1.200 | 40 | 0.000015 | |
| 6 | 0.20 (1.0 µmol/L) | 0.70 (5.9 µmol/L) | 0.30 (2.5 µmol/L) | −1.200 | 70 | 0.000008 | |
| Simplex | |||||||
| B1 | 0.94 (4.5 µmol/L) | 0.58 (4.9 µmol/L) | 0.28 (2.3 µmol/L) | −1.400 | 82 | 0.005867 | W13 |
| B2 | 0.64 (3.1 µmol/L) | 0.83 (7.0 µmol/L) | 0.01 (0.1 µmol/L) | −1.540 * | 33 | 0.003432 | W8, W10, W12 |
| B3 | 1.31 (6.3 µmol/L) | 1.08 (9.1 µmol/L) | 0.44 (3.6 µmol/L) | −1.480 | 42 | 0.001847 | W6 |
| B4 | 1.19 (5.7 µmol/L) | 0.60 (5.1 µmol/L) | 0.39 (3.2 µmol/L) | −1.372 | 77 | 0.018896 | |
| B5 | 1.15 (5.5 µmol/L) | 1.22 (10.3 µmol/L) | 0.57 (4.7 µmol/L) | −1.601 * | 109 | 0.005121 | W9 |
| B6 | 0.58 (2.8 µmol/L) | 0.49 (4.1 µmol/L) | 0.38 (3.1 µmol/L) | −1.429 | 114 | 0.006527 | W14 |
| B7 | 1.00 (4.8 µmol/L) | 0.79 (6.7 µmol/L) | −0.15 ** (−1.2 µmol/L) | −1.380 | 76 | 0.013090 | |
| B8 | 1.31 (6.3 µmol/L) | 0.64 (5.7 µmol/L) | 0.64 (5.3 µmol/L) | −1.332 | 151 | 0.001571 | |
| B9 | 0.59 (2.8 µmol/L) | 0.10 (0.8 µmol/L) | −0.14 ** (−1.1 µmol/L) | −1.332 | 43 | 0.003460 | W11 |
| B10 | 1.08 (5.2 µmol/L) | 0.19 (1.6 µmol/L) | 0.41 (3.4 µmol/L) | −1.265 | 124 | 0.000383 | |
| B11 | 0.94 (4.5 µmol/L) | 0.80 (6.7 µmol/L) | 0.30 (2.5 µmol/L) | −1.437 | 85 | 0.046550 | |
| B12 | 1.00 (4.8 µmol/L) | 0.60 (5.1 µmol/L) | 0.34 (2.8 µmol/L) | −1.380 | 100 | 0.111153 | |
| B13 | 0.94 (4.5 µmol/L) | 0.67 (5.6 µmol/L) | 0.28 (2.3 µmol/L) | −1.399 | 93 | 0.001522 | |
| B14 | 1.13 (5.4 µmol/L) | 0.72 (6.1 µmol/L) | 0.23 (1.9 µmol/L) | −1.385 | 76 | 0.001275 | |
| Electrode No. | Electrode Designation | Zn(II) | Cd(II) | Pb(II) | |||
|---|---|---|---|---|---|---|---|
| LOD [µg/L] | LOQ [µg/L] | LOD [µg/L] | LOQ [µg/L] | LOD [µg/L] | LOQ [µg/L] | ||
| Factorial Design | |||||||
| 1 | 0.80Bi0.70Sn0.80Sb | 2.5 | 3.5 | 0.5 | 1.5 | 1.0 | 3.0 |
| 2 | 0.60Bi0.80Sn0.30Sb | 1.5 | 3.5 | 1.0 | 3.0 | 1.5 | 4.5 |
| 3 | 0.70Bi0.20Sn0.70Sb | 2.5 | 3.0 | 0.5 | 1.5 | 1.0 | 3.0 |
| 4 | 0.80Bi0.30Sn0.20Sb | 2.0 | 3.0 | 1.0 | 3.0 | 1.0 | 4.0 |
| 5 | 0.20Bi0.80Sn0.80Sb | 3.5 | 5.5 | 2.0 | 3.5 | 2.0 | 4.5 |
| 6 | 0.20Bi0.70Sn0.30Sb | 3.0 | 3.5 | 1.0 | 1.5 | 1.0 | 2.5 |
| 7 | 0.30Bi0.30Sn0.60Sb | 2.0 | 3.0 | 2.5 | 5.5 | 3.0 | 6.5 |
| 8 | 0.20Bi0.20Sn0.20Sb | 1.0 | 2.5 | 1.5 | 3.0 | 1.0 | 2.5 |
| Simplex | |||||||
| 1 | 0.94Bi0.58Sn0.28Sb | 2.5 | 3.7 | 0.7 | 1.7 | 1.2 | 3.2 |
| 2 | 0.64Bi0.83Sn0.01Sb | 2.7 | 4.5 | 1.7 | 4.0 | 1.5 | 4.0 |
| 3 | 1.31Bi1.08Sn0.44Sb | 4.0 | 5.0 | 1.0 | 2.7 | 1.5 | 4.7 |
| 4 | 1.19Bi0.60Sn0.39Sb | 2.5 | 3.5 | 0.5 | 1.2 | 1.0 | 2.5 |
| 5 | 1.15Bi1.22Sn0.57Sb | 3.2 | 4.2 | 0.5 | 1.2 | 1.0 | 2.5 |
| 6 | 0.58Bi0.49Sn0.38Sb | 1.7 | 2.5 | 0.5 | 1.2 | 0.5 | 1.5 |
| 7 | 1.00Bi0.79Sn | 1.5 | 2.5 | 0.7 | 1.5 | 0.7 | 2.7 |
| 8 | 1.31Bi0.64Sn0.64Sb | 2.5 | 3.0 | 0.5 | 1.0 | 1.0 | 2.2 |
| 9 | 0.59Bi0.10Sn | 1.7 | 3.2 | 0.7 | 2.0 | 1.2 | 3.5 |
| 10 | 1.08Bi0.19Sn0.41Sb | 3.2 | 3.7 | 0.5 | 1.0 | 1.0 | 2.5 |
| 11 | 0.94Bi0.80Sn0.30Sb | 2.5 | 3.5 | 0.7 | 1.7 | 1.2 | 2.7 |
| 12 | 1.00Bi0.60Sn0.34Sb | 3.0 | 4.0 | 0.5 | 1.2 | 1.2 | 2.7 |
| 13 | 0.94Bi0.67Sn0.28Sb | 2.5 | 3.2 | 0.5 | 1.5 | 1.0 | 2.7 |
| 14 | 1.13Bi0.72Sn0.23Sb | 2.0 | 2.7 | 0.7 | 1.7 | 1.5 | 3.5 |
| Electrode No. | Electrode Designation | Zn(II) | Cd(II) | Pb(II) | |
|---|---|---|---|---|---|
| γ [µg/L] | RSD [%] | RSD [%] | RSD [%] | ||
| Factorial Design | |||||
| 1 | 0.80Bi0.70Sn0.80Sb | 117.5 | 8.3 | 1.8 | 4.0 |
| 2 | 0.60Bi0.80Sn0.30Sb | 59.1 | 14.8 | 3.7 | 9.6 |
| 3 | 0.70Bi0.20Sn0.70Sb | 78.7 | 4.6 | 7.2 | 7.2 |
| 4 | 0.80Bi0.30Sn0.20Sb | 10.0 | 17.6 | 2.9 | 4.1 |
| 5 | 0.20Bi0.80Sn0.80Sb | 49.3 | 6.6 | 2.1 | 5.3 |
| 6 | 0.20Bi0.70Sn0.30Sb | 14.8 | 11.8 | 8.0 | 12.4 |
| 7 | 0.30Bi0.30Sn0.60Sb | 5.0 (72.2 µg/L) | 5.3 (199.5 µg/L) | 2.1 (199.5 µg/L) | |
| 8 | 0.20Bi0.20Sn0.20Sb | 43.4 | 2.3 | 2.3 | 1.4 |
| Simplex | |||||
| 1 | 0.94Bi0.58Sn0.28Sb | 141.6 | 1.5 | 7.2 | 4.3 |
| 2 | 0.64Bi0.83Sn0.01Sb | 39.3 | 26.8 | 18.0 | 10.4 |
| 3 | 1.31Bi1.08Sn0.44Sb | 126.2 | 13.0 | 5.9 | 3.9 |
| 4 | 1.19Bi0.60Sn0.39Sb | 141.6 | 6.7 | 1.0 | 6.0 |
| 5 | 1.15Bi1.22Sn0.57Sb | 121.4 | 9.5 | 2.0 | 7.4 |
| 6 | 0.58Bi0.49Sn0.38Sb | 54.2 | 13.2 | 2.8 | 20.7 |
| 7 | 1.00Bi0.79Sn | 29.6 | 9.3 | 13.8 | 12.0 |
| 8 | 1.31Bi0.64Sn0.64Sb | 58.8 | 5.3 | 8.6 | 16.3 |
| 9 | 0.59Bi0.10Sn | 29.6 | 2.2 | 2.0 | 17.3 |
| 10 | 1.08Bi0.19Sn0.41Sb | 78.1 | 3.2 | 7.9 | 17.3 |
| 11 | 0.94Bi0.80Sn0.30Sb | 97.4 | 3.8 | 2.4 | 6.8 |
| 12 | 1.00Bi0.60Sn0.34Sb | 135.7 | 8.1 | 2.0 | 7.6 |
| 13 | 0.94Bi0.67Sn0.28Sb | 126.2 | 2.8 | 6.7 | 6.2 |
| 14 | 1.13Bi0.72Sn0.23Sb | 135.7 | 13.4 | 20.1 | 2.2 |
| In Situ FE | 1.00Bi0.60Sn0.34Sb | 1.94Bi | 1.94Sn | 1.94Sb |
|---|---|---|---|---|
| Zn(II) | ||||
| Linearity [µg/L] | 135.7–225.6 | 5.0–82.9 | ** | 10.0–64.9 |
| Slope [µAL/µg] | 1.157 | 0.830 | ** | 0.932 |
| LOD [µg/L] | 3.0 | 3.2 | 39.2 | 1.0 |
| LOQ [µg/L] | 4.0 | 3.7 | 49.0 | 1.5 |
| Re [%] | 50.3 | 105.6 | ** | 28.1 |
| (135.7 µg/L) | (29.6 µg/L) | (10.0 µg/L) | ||
| RSD [%] | 36.1 | 19.7 | ** | 43.1 |
| Cd(II) | ||||
| Linearity [µg/L] | 4.0–19.8 | 10.0–154.8 | 19.9–225.6 | 16.9–159.5 |
| 39.3–225.6 | ||||
| Slope [µAL/µg] | 0.379 | 0.657 | 0.323 | 0.479 |
| 0.691 | ||||
| LOD [µg/L] | 0.5 | 0.5 | * | * |
| LOQ [µg/L] | 1.2 | 1.0 | * | * |
| Re [%] | 98.3 | 89.7 | 80.2 | 60.5 |
| (135.7 µg/L) | (29.6 µg/L) | (63.2 µg/L) | (55.3 µg/L) | |
| RSD [%] | 9.5 | 10.3 | 33.4 | 17.1 |
| Pb(II) | ||||
| Linearity [µg/L] | 19.8–386.8 | 29.6–341.3 | 34.4–341.3 | 55.3–1130.9 |
| Slope [µAL/µg] | 0.333 | 0.326 | 0.250 | 0.060 |
| LOD [µg/L] | 1.2 | 1.2 | 7.4 | 7.4 |
| LOQ [µg/L] | 2.7 | 3.7 | 10.3 | 12.4 |
| Re [%] | 104.5 | 67.1 | 52.6 | 68.8 |
| (135.7 µg/L) | (29.6 µg/L) | (63.2 µg/L) | (55.3 µg/L) | |
| RSD [%] | 8.7 | 12.6 | 41.2 | 31.6 |
| OCcombined | 0.111153 | 0.009410 | *** | *** |
| Possible Interferent | Mass Concentration Ratio Zn(II):Interferent | Mass Concentration Ratio Cd(II):Interferent | Mass Concentration Ratio Pb(II):Interferent | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:1 | 1:10 | 1:100 | 1:1 | 1:10 | 1:100 | 1:1 | 1:10 | 1:100 | |
| Cu(II) | −90.9 | −99.6 | * | −58.4 | −99.3 | * | −49.5 | −79.3 | * |
| Mg(II) | −3.5 | −9.0 | −42.1 | −1.7 | −4.2 | −32.7 | −4.4 | −9.7 | −40.3 |
| As(III) | 3.4 | −6.9 | −14.5 | 5.5 | 2.5 | 5.5 | −7.4 | −16.5 | −21.0 |
| Fe(II) | −28.0 | −91.4 | * | −14.6 | −10.5 | −41.7 | −22.3 | −36.4 | * |
| Ca(II) | −8.8 | −14.7 | −7.3 | −2.3 | −6.6 | −17.5 | −5.6 | −13.7 | −26.8 |
| K(I) | 10.0 | 12.3 | −28.6 | 3.6 | 4.6 | −19.0 | −3.5 | −7.9 | −31.8 |
| Cl− | 18.0 | 16.9 | −21.8 | 2.5 | 5.1 | −18.8 | −6.5 | −12.0 | −36.7 |
| SO42− | 7.1 | 9.6 | −30.3 | −0.1 | 0.1 | −22.8 | −8.9 | −15.1 | −38.4 |
| NO3− | 10.3 | 11.4 | −23.5 | −0.4 | −1.2 | −27.0 | −3.7 | −10.7 | −39.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finšgar, M.; Jezernik, K. The Use of Factorial Design and Simplex Optimization to Improve Analytical Performance of In Situ Film Electrodes. Sensors 2020, 20, 3921. https://doi.org/10.3390/s20143921
Finšgar M, Jezernik K. The Use of Factorial Design and Simplex Optimization to Improve Analytical Performance of In Situ Film Electrodes. Sensors. 2020; 20(14):3921. https://doi.org/10.3390/s20143921
Chicago/Turabian StyleFinšgar, Matjaž, and Klara Jezernik. 2020. "The Use of Factorial Design and Simplex Optimization to Improve Analytical Performance of In Situ Film Electrodes" Sensors 20, no. 14: 3921. https://doi.org/10.3390/s20143921