Abstract
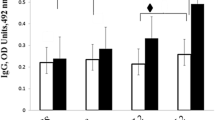
Since Brucella infection mostly occurs through the mucosal surfaces, immune response induced by vaccine that is delivered by a way of mucosal route can be drastically enhanced to control the brucellosis. Omp31is the major outer membrane protein of Brucella, and is considered as a protective antigen against Brucella infection. Accordingly, Lactococcus lactis has been used as an antigen-delivering vector to develop a vaccine-induced mucosal response for having a safer vaccination against brucellosis. A designed omp31 gene fused to the usp45 signal peptide and M6 cell wall anchor was sub cloned in the pNZ7021 expression vector, and a recombinant L. lactis displaying Omp31 was constructed. Omp31 protein expression was confirmed using Western blotting and immunofluorescence analysis. Animals were orally and intraperitoneally immunized with live or killed L. lactis expressing Omp31, respectively. The humoral and cellular immune responses were evaluated by measuring the specific cytokines and antibodies. sIgA, serum IgA, IgM, and total IgG antibodies significantly increased in the mice immunized with live recombinant L. lactis expressing Omp31 and also serum IgM, and total IgG antibodies significantly increased in mice immunized with killed recombinant L. lactis expressing Omp31. Among IgG subtypes, IgG2a response was significantly higher in both groups compared to IgG1. In mice groups immunized with recombinant L. lactis, the IFN-γ and IL-10 level elevated; however, there was no change in the level of IL-4. These results indicated that recombinants L. lactis induce both humoral and cellular immune responses in mice, and also vaccines based on L. lactis-derived live carriers are promising interventions against Brucella melitensis infections.








Similar content being viewed by others
References
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infect Dis 6(2):91–99. https://doi.org/10.1016/S1473-3099(06)70382-6
World Health O, United Kingdom. Dept. for International Development. Animal Health P, Food, Agriculture Organization of the United N, World Organisation for Animal H (2006) The control of neglected zoonotic diseases : a route to poverty alleviation : report of a joint WHO/DFID-AHP meeting, 20 and 21 September 2005, WHO headquarters, Geneva, with the participation of FAO and OIE. World Health Organization, Geneva http://apps.who.int/iris/handle/10665/43485. Accessed 10 Mar 2020
Seyedalizadeh N, Alesheikh A, Ahmadkhani M (2019) Spatio-statistical modeling of human brucellosis using environmental parameters: a case study of northern Iran. ISPRS Archives 42:969–973. https://doi.org/10.5194/isprs-archives-XLII-4-W18-969-2019
Moreno E, Moriyón I (2002) Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc Natl Acad Sci U S A 99(1):1–3. https://doi.org/10.1073/pnas.022622699
Pappas G, Akritidis N, Bosilkovski M, Tsianos E (2005) the b. melitensis genome. N Engl J Med 352:2325–2336. https://doi.org/10.1056/NEJMra050570
Guzmán-Verri C, Manterola L, Sola-Landa A, Parra A, Cloeckaert A, Garin J, Gorvel J-P, Moriyón I, Moreno E, Lopez-Goñi I (2002) The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc Natl Acad Sci U S A 99(19):12375–12380. https://doi.org/10.1073/pnas.192439399
Boschiroli ML, Foulongne V, O'Callaghan D (2001) Brucellosis: a worldwide zoonosis. Curr Opin Microbiol 4(1):58–64. https://doi.org/10.1016/s1369-5274(00)00165-x
Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ (1989) Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol 143(10):3330–3337
Olsen SC (2013) Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech 32(1):207–217. https://doi.org/10.20506/rst.32.1.2201
Guilloteau LA, Laroucau K, Vizcaı́no N, Jacques I, Dubray G (1999) Immunogenicity of recombinant Escherichia coli expressing the omp31 gene of Brucella melitensis in BALB/c mice. Vaccine 17(4):353–361. https://doi.org/10.1016/S0264-410X(98)00205-9
Vitry MA, Hanot Mambres D, De Trez C, Akira S, Ryffel B, Letesson JJ, Muraille E (2014) Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J Immunol 192(8):3740–3752. https://doi.org/10.4049/jimmunol.1302561
Nazifi N, Tahmoorespur M, Sekhavati MH, Haghparast A, Behroozikhah AM (2019) In vivo immunogenicity assessment and vaccine efficacy evaluation of a chimeric tandem repeat of epitopic region of OMP31 antigen fused to interleukin 2 (IL-2) against Brucella melitensis in BALB/c mice. BMC Vet Res 15(1):1–11. https://doi.org/10.1186/s12917-019-2074-7
DelVecchio VG, Alefantis T, Ugalde RA, Comerci D, Marchesini MI, Khan A, Lubitz W, Mujer CV (2006) Identification of protein candidates for developing bacterial ghost vaccines against Brucella. Methods Biochem Anal 49:363–377. https://doi.org/10.1002/0471973165.ch19
Cassataro J, Velikovsky CA, de la Barrera S, Estein SM, Bruno L, Bowden R, Pasquevich KA, Fossati CA, Giambartolomei GH (2005) A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun 73(10):6537–6546. https://doi.org/10.1128/IAI.73.10.6537-6546.2005
Sáez D, Fernández P, Rivera A, Andrews E, Oñate A (2012) Oral immunization of mice with recombinant Lactococcus lactis expressing Cu, Zn superoxide dismutase of Brucella abortus triggers protective immunity. Vaccine 30(7):1283–1290. https://doi.org/10.1016/j.vaccine.2011.12.088
Miyoshi A, Bermúdez-Humarán LG, Ribeiro LA, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2006) Heterologous expression of Brucella abortus GroEL heat-shock protein in Lactococcus lactis. Microb Cell Factories 5(1):14. https://doi.org/10.1186/1475-2859-5-14
Liu J-K, Hou X-L, Wei C-H, Yu L-Y, He X-J, Wang G-H, Lee J-S, Kim C-J (2009) Induction of immune responses in mice after oral immunization with recombinant Lactobacillus casei strains expressing enterotoxigenic Escherichia coli F41 fimbrial protein. Appl Environ Microbiol 75(13):4491–4497. https://doi.org/10.1128/AEM.02672-08
Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189(8):3256–3270. https://doi.org/10.1128/JB.01768-06
Ribeiro LA, Azevedo V, Le Loir Y, Oliveira SC, Dieye Y, Piard JC, Gruss A, Langella P (2002) Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl Environ Microbiol 68(2):910–916. https://doi.org/10.1128/AEM.68.2.910-916.2002
Bahey-El-Din M, Gahan CG (2011) Lactococcus lactis-based vaccines: current status and future perspectives. Human vaccines 7(1):106–109. https://doi.org/10.4161/hv.7.1.13631
Bahey-El-Din M (2012) Lactococcus lactis-based vaccines from laboratory bench to human use: an overview. Vaccine 30(4):685–690. https://doi.org/10.1016/j.vaccine.2011.11.098
Berlec A, Ravnikar M, Štrukelj B (2012) Lactic acid bacteria as oral delivery systems for biomolecules. Pharmazie 67(11):891–898. https://doi.org/10.1691/ph.2012.1705
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68. https://doi.org/10.1016/0076-6879(90)82008-p
Yousefi S, Sekhavati MH, Tahmoorespur M, Abbassi-Daloii M (2016) Cloning and molecular characterization of Omp31 gene from Brucella melitensis rev 1 strain. Arch Razi Inst 71(2):117–124. https://doi.org/10.22034/ARI.2016.106450
Bohlul E, Hasanlou F, Taromchi AH, Nadri S (2019) TRAIL-expressing recombinant Lactococcus lactis induces apoptosis in human colon adenocarcinoma SW 480 and HCT 116 cells. J Appl Microbiol 126(5):1558–1567. https://doi.org/10.1111/jam.14237
Wegkamp A, van Oorschot W, de Vos WM, Smid EJ (2007) Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl Environ Microbiol 73(8):2673–2681. https://doi.org/10.1128/AEM.02174-06
Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, Galleri L, Spagnuolo I, Steidler L, Van Huynegem K, Demetter P, Wasserfall C, Atkinson MA, Dotta F, Rottiers P, Gysemans C, Mathieu C (2012) Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest 122(5):1717–1725. https://doi.org/10.1172/JCI60530
Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L (2006) A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol 4(6):754–759. https://doi.org/10.1016/j.cgh.2006.03.028
Cortes-Perez NG, Azevedo V, Alcocer-González JM, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, Gruss A, Langella P, Bermúdez-Humarán LG (2005) Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J Drug Target 13(2):89–98. https://doi.org/10.1080/10611860400024219
Stabel TJMJ, Tabatabai LB, Wannemuehler MJ (1990) Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun 58(7):2048–2055. https://doi.org/10.1128/IAI.58.7.2048-2055.1990
Villena J, Medina M, Raya R, Alvarez S (2008) Oral immunization with recombinant Lactococcus lactis confers protection against respiratory pneumococcal infection. Can J Microbiol 54(10):845–853. https://doi.org/10.1139/W08-077
Pontes DSDF, Ribeiro LA, Miyoshi A, LeLoir Y, Gruss A, Oliveira SC, Langella P, Azevedo V (2003) Induction of partial protection in mice after oral administration of Lactococcus lactis producing Brucella abortus L7/L12 antigen. J Drug Target 11(8–10):489–493. https://doi.org/10.1080/10611860410001670035
Cassataro JES, Pasquevich KA, Velikovsky CA, de la Barrera S, Bowden R, Fossati CA, Giambartolomei GH (2005) Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infect Immun 73(12):8079–8088. https://doi.org/10.1128/iai.73.12.8079-8088.2005
Abbassi-Daloii T, Yousefi SSM, Tahmoorespur M (2018) Impact of heat shock protein 60KD in combination with outer membrane proteins on immune response against Brucella melitensis. APMIS 126(1):65–75. https://doi.org/10.1111/apm.12778
Gupta VKRG, Harms J, Splitter G (2012) Invasive Escherichia coli vaccines expressing Brucella melitensis outer membrane proteins 31 or 16 or periplasmic protein BP26 confer protection in mice challenged with B. melitensis. Vaccine 30(27):4017–4022. https://doi.org/10.1016/j.vaccine.2012.04.036
Doosti A, Ghasemi-Dehkordi P, Javadi GR, Sardari S, Shokrgozar MA (2009) DNA vaccine encoding the Omp31 gene of Brucella melitensis induces protective immunity in BALB/c mice. Res J Biol Sci 4(1):126–131 rjbsci.2009.126.131
Unkeless JC, Scigliano E, Freedman VH (1988) Structure and function of human and murine receptors for IgG. Annu Rev Immunol 6(1):251–281. https://doi.org/10.1146/annurev.iy.06.040188.001343
Shibaki AKS (2002) Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund's adjuvant. Exp Dermatol 11(2):126–134. https://doi.org/10.1034/j.1600-0625.2002.110204.x
Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL (2001) Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103(4):511–518. https://doi.org/10.1046/j.1365-2567.2001.01258.x
Zhan Y, Liu Z, Cheers C (1996) Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect Immun 64(7):2782–2786. https://doi.org/10.1128/IAI.64.7.2782-2786.1996
Clausse M, Diaz AG, Ibanez AE, Cassataro J, Giambartolomei GH, Estein SM (2014) Evaluation of the efficacy of outer membrane protein 31 vaccine formulations for protection against Brucella canis in BALB/c mice. Clin Vaccine Immunol 21(12):1689–1694. https://doi.org/10.1128/cvi.00527-14
Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J (2004) Induction of IgG2a class switching in B cells by IL-27. J Immunol 173(4):2479–2485. https://doi.org/10.4049/jimmunol.173.4.2479
Tewari AK, Kurup SP, Baidya S, Barta JR, Sharma B (2015) Protective antibody and cytokine responses in mice following immunization with recombinant beta-tubulin and subsequent Trypanosoma evansi challenge. Parasites Vectors 8(1):580. https://doi.org/10.1186/s13071-015-1189-3
Ghasemi A, Jeddi-Tehrani M, Mautner J, Salari MH, Zarnani A-H (2015) Simultaneous immunization of mice with Omp31 and TF provides protection against Brucella melitensis infection. Vaccine 33(42):5532–5538. https://doi.org/10.1016/j.vaccine.2015.09.013
Mathers AR, Cuff CF (2004) Role of Interleukin-4 (IL-4) and IL-10 in serum immunoglobulin G antibody responses following mucosal or systemic Reovirus infection. J Virol 78(7):3352–3360. https://doi.org/10.1128/jvi.78.7.3352-3360.2004
Mosmann TR, Coffman R (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7(1):145–173. https://doi.org/10.1146/annurev.iy.07.040189.001045
Aliramaei MR, Khorasgani MR, Rahmani MR, Esfahani SHZ, Emamzadeh R (2020) Expression of Helicobacter pylori CagL gene in Lactococcus lactis MG1363 and evaluation of its immunogenicity as an oral vaccine in mice. Microb Pathog 142:103926. https://doi.org/10.1016/j.micpath.2019.103926
Liu X, Qi L, Lv J, Zhang Z, Zhou P, Ma Z, Wang Y, Zhang Y, Pan L (2020) The immune response to a recombinant Lactococcus lactis oral vaccine against foot-and-mouth disease virus in mice. Biotechnol Lett:1–11. https://doi.org/10.1007/s10529-020-02900-6
Acknowledgments
The present study was supported by the grant from Zanjan University of Medical Sciences (grant NO. A- 12-873-7).
The authors thank Dr. Negar Seyed, Dr.Yeganeh Talebkhan at Pasteur Institute of Iran and Dr.Narges Nazifi, Dr.Soheil Yousefi at Ferdowsi University of Mashhad for their technical guidance and constant support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Amirhossein Taromchi and Hoda Shirdast: Material preparation; Methodology: Hoda Shirdast and Fatemeh Ebrahimzadeh; data collection and analysis: Hoda Shirdast and Amirhossein Taromchi; Rabbit immunization: Esmat Mirabzadeh; Mice immunization: Hoda Shirdast and Keivan Nedaei; Writing - original draft preparation: Hoda Shirdast; Writing - review and editing: Amirhossein Taromchi, Yousef Mortazavi, Mohammad Hadi Sekhavati and Abdolreza Esmaeilzadeh; Supervision: Amirhossein Taromchi. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The study was approved by the Animal Experimentation Ethics Committee of Zanjan University of Medical Sciences (ZUMS.REC.1396.146).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shirdast, H., Ebrahimzadeh, F., Taromchi, A.H. et al. Recombinant Lactococcus Lactis Displaying Omp31 Antigen of Brucella melitensis Can Induce an Immunogenic Response in BALB/c Mice. Probiotics & Antimicro. Prot. 13, 80–89 (2021). https://doi.org/10.1007/s12602-020-09684-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09684-1