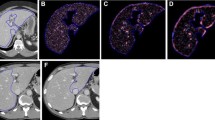
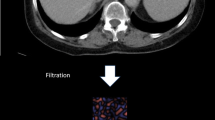
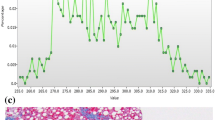
Abstract
Purpose
To evaluate feasibility of computer tomography texture analysis (CTTA) at different energy level using dual-energy spectral detector CT for liver fibrosis.
Materials and methods
Eighty-seven patients who underwent a spectral CT examination and had a reference standard of liver fibrosis (histopathologic findings, n = 61, or clinical findings for normal, n = 26) were included. Mean gray-level intensity, mean number of positive pixels (MPP), entropy, skewness, and kurtosis using commercially available software (TexRAD) were compared at different energy levels. Optimal CTTA parameter cutoffs to diagnose liver fibrosis were evaluated. CTTA parameters at different energy levels correlated with liver fibrosis. The association of CTTA parameters with energy level was evaluated.
Results
Mean gray-level intensity, skewness, kurtosis, and entropy showed significant differences between patients with and without clinically significant hepatic fibrosis (P < 0.05). Mean gray-level intensity at 50 keV was significantly positively correlated with liver fibrosis (ρ = 0.502, P < 0.001). To diagnose stages F2–F4, entropy and mean gray-level intensity at low keV level showed the largest area under the curve (AUC; 0.79 and 0.79). Estimated marginal means (EMMs) of mean gray-level intensity showed prominent differences at low energy levels.
Conclusion
CTTA parameters from different keV levels demonstrated meaningful accuracy for diagnosis of liver fibrosis or clinically significant hepatic fibrosis.





Similar content being viewed by others
References
Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756–62.
Lee SS, Byoun YS, Jeong SH, Kim YM, Gil H, Min BY, et al. Type and cause of liver disease in korea: single-center experience, 2005–2010. Clin Mol Hepatol. 2012;18:309–15.
World Health Organization. Global hepatitis report 2017. Washington: World Health Organization; 2017.
Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38:38–533.
Horowitz JM, Venkatesh SK, Ehman RL, Jhaveri K, Kamath P, Ohliger MA, et al. Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (New York). 2017;42:2037–53.
Akkaya HE, Erden A, Kuru Oz D, Unal S, Erden I. Magnetic resonance elastography: basic principles, technique, and clinical applications in the liver. Diagn Interv Radiol. 2018;24:328–35.
Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500.
Park HJ, Lee SS, Park B, Yun J, Sung YS, Shim WH, et al. Radiomics analysis of gadoxetic acid-enhanced MRI for staging liver fibrosis. Radiology. 2019;290:380–7.
Low G, Kruse SA, Lomas DJ. General review of magnetic resonance elastography. World J Radiol. 2016;8:59–72.
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. Ct texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics. 2017;37:1483–503.
Hanania AN, Bantis LE, Feng Z, Wang H, Tamm EP, Katz MH, et al. Quantitative imaging to evaluate malignant potential of ipmns. Oncotarget. 2016;7:85776–84.
Hu Y, Liang Z, Song B, Han H, Pickhardt PJ, Zhu W, et al. Texture feature extraction and analysis for polyp differentiation via computed tomography colonography. IEEE Trans Med Imaging. 2016;35:1522–31.
Raman SP, Chen Y, Schroeder JL, Huang P, Fishman EK. Ct texture analysis of renal masses: pilot study using random forest classification for prediction of pathology. Acad Radiol. 2014;21:1587–96.
Raman SP, Schroeder JL, Huang P, Chen Y, Coquia SF, Kawamoto S, et al. Preliminary data using computed tomography texture analysis for the classification of hypervascular liver lesions: generation of a predictive model on the basis of quantitative spatial frequency measurements—a work in progress. J Comput Assist Tomogr. 2015;39:383–95.
Lubner MG, Malecki K, Kloke J, Ganeshan B, Pickhardt PJ. Texture analysis of the liver at mdct for assessing hepatic fibrosis. Abdom Radiol (New York). 2017;42:2069–78.
Baliyan V, Kordbacheh H, Parameswaran B, Ganeshan B, Sahani D, Kambadakone A. Virtual monoenergetic imaging in rapid kvp-switching dual-energy ct (dect) of the abdomen: impact on ct texture analysis. Abdom Radiol (New York). 2018;43:2693–701.
Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, et al. Dual-energy multidetector ct: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30:1037–55.
Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607.
Chung SR, Lee SS, Kim N, Yu ES, Kim E, Kuhn B, et al. Intravoxel incoherent motion MRI for liver fibrosis assessment: a pilot study. Acta Radiol. 2015;56:1428–36.
Ganeshan B, Miles KA, Young RC, Chatwin CR. Hepatic enhancement in colorectal cancer: texture analysis correlates with hepatic hemodynamics and patient survival. Acad Radiol. 2007;14:1520–30.
Ganeshan B, Miles KA, Young RC, Chatwin CR. In search of biologic correlates for liver texture on portal-phase ct. Acad Radiol. 2007;14:1058–68.
Bandula S, Punwani S, Rosenberg WM, Jalan R, Hall AR, Dhillon A, et al. Equilibrium contrast-enhanced ct imaging to evaluate hepatic fibrosis: Initial validation by comparison with histopathologic sampling. Radiology. 2015;275:136–43.
Ganeshan B, Miles KA, Young RC, Chatwin CR. Texture analysis in non-contrast enhanced ct: impact of malignancy on texture in apparently disease-free areas of the liver. Eur J Radiol. 2009;70:101–10.
Daginawala N, Li B, Buch K, Yu H, Tischler B, Qureshi MM, et al. Using texture analyses of contrast enhanced ct to assess hepatic fibrosis. Eur J Radiol. 2016;85:511–7.
Lubner MG, Jones D, Kloke J, Said A, Pickhardt PJ. Ct texture analysis of the liver for assessing hepatic fibrosis in patients with hepatitis c virus. Br J Radiol. 2019;92:20180153.
Varghese BA, Cen SY, Hwang DH, Duddalwar VA. Texture analysis of imaging: what radiologists need to know. Am J Roentgenol. 2019;212:520–8.
Ganeshan B, Abaleke S, Young RC, Chatwin CR, Miles KA. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: Initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging. 2010;10:137–43.
Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: Ct texture as a predictive biomarker. Radiology. 2011;261:165–71.
Miles KA, Ganeshan B, Hayball MP. Ct texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging. 2013;13:400–6.
Rassouli N, Etesami M, Dhanantwari A, Rajiah P. Detector-based spectral ct with a novel dual-layer technology: principles and applications. Insights Imaging. 2017;8:589–98.
Rassouli N, Chalian H, Rajiah P, Dhanantwari A, Landeras L. Assessment of 70-kev virtual monoenergetic spectral images in abdominal ct imaging: a comparison study to conventional polychromatic 120-kvp images. Abdom Radiol (New York). 2017;42:2579–86.
Baron RL, Gore R. Diffuse liver disease. Textbook of gastrointestinal radiology. 2nd ed. Philadelphia: Saunders; 2000. p. 1590–1638.
Ohkoshi S, Hirono H, Watanabe K, Hasegawa K, Kamimura K, Yano M. Natural regression of fibrosis in chronic hepatitis b. World J Gastroenterol. 2016;22:5459–66.
Ros PR, Mortele KJ. Diffuse liver disease. Clin Liver Dis. 2002;6:181–201.
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56.
Sofue K, Tsurusaki M, Mileto A, Hyodo T, Sasaki K, Nishii T, et al. Dual-energy computed tomography for non-invasive staging of liver fibrosis: accuracy of iodine density measurements from contrast-enhanced data. Hepatol Res. 2018;48:1008–199.
Murray N, Darras KE, Walstra FE, Mohammed MF. Dual-energy ct in evaluation of the acute abdomen. Radiographics. 2019;39:264–86.
Choi KJ, Jang JK, Lee SS, Sung YS, Shim WH, Kim HS. Development and validation of a deep learning system for staging liver fibrosis by using contrast agent-enhanced ct images in the liver. Radiology. 2018;289:688–97.
Choi IY, Yeom SK. Feasibility of using computed tomography texture analysis parameters as imaging biomarkers for predicting risk grade of gastrointestinal stromal tumors: comparison with visual inspection. Abdom Radiol (NY). 2019;44:2346–56.
Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumor heterogeneity by ct texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol. 2013;82:342–8.
Ito E, Sato K, Yamamoto R, Sakamoto K, Urakawa H, Yoshimitsu K. Usefulness of iodine-blood material density images in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine dual-energy liver ct protocol equilibrium phase data: preliminary experience. Jpn J Radiol. 2020;38:365–73.
Shinagawa Y, Sakamoto K, Sato K, Ito E, Urakawa H, Yoshimitsu K. Usefulness of new subtraction algorithm in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine liver ct protocol equilibrium phase data: preliminary experience. Eur J Radiol. 2018;103:99–104.
Acknowledgements
This research was supported by Korea University Ansan Hospital Grant (O1801331).
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The scientific guarantor of this publication is Hwan Hoon Chung. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Choi, B., Choi, I.Y., Cha, S.H. et al. Feasibility of computed tomography texture analysis of hepatic fibrosis using dual-energy spectral detector computed tomography. Jpn J Radiol 38, 1179–1189 (2020). https://doi.org/10.1007/s11604-020-01020-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-01020-5