Abstract
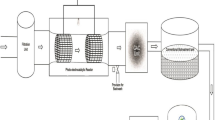
This study evaluated the feasibility of electrochemical hydrogen peroxide (H2O2) production with gas diffusion electrode (GDE) for decentralized water treatment. Carbon black-polytetrafluoroethylene GDEs were prepared and tested in a continuous flow electrochemical cell for H2O2 production from oxygen reduction. Results showed that because of the effective oxygen transfer in GDEs, the electrode maintained high apparent current efficiencies (ACEs,>80%) for H2O2 production over a wide current density range of 5–400 mA/cm2, and H2O2 production rates as high as ∼202 mg/h/cm2 could be obtained. Long-term stability test showed that the GDE maintained high ACEs (>85%) and low energy consumption (< 10 kWh/kg H2O2) for H2O2 production for 42 d (∼1000 h). However, the ACEs then decreased to ∼70% in the following 4 days because water flooding of GDE pores considerably impeded oxygen transport at the late stage of the trial. Based on an electrode lifetime of 46 days, the overall cost for H2O2 production was estimated to be ∼0.88 $/kg H2O2 including an electricity cost of 0.61 $/kg and an electrode capital cost of 0.27 $/kg. With a 9 cm2 GDE and 40 mA/cm2 current density, ∼2–4 mg/L of H2O2 could be produced on site for the electro-peroxone treatment of a 1.2 m3/d groundwater flow, which considerably enhanced ibuprofen abatement compared with ozonation alone (∼43%–59% vs. 7%). These findings suggest that electrochemical H2O2 production with GDEs holds great promise for the development of compact treatment technologies for decentralized water treatment at a household and community level.

Similar content being viewed by others
References
Alcaide F, Álvarez G, Guelfi D R V, Brillas E, Sirés I (2020). A stable CoSP/MWCNTs air-diffusion cathode for the photoelectro-Fenton degradation of organic pollutants at pre-pilot scale. Chemical Engineering Journal, 379: 122417
Assumpção M H M T, De Souza R F B, Rascio D C, Silva J C M, Calegaro M L, Gaubeur I, Paixao T R L C, Hammer P, Lanza M R V, Santos M C (2011). A comparative study of the electrogeneration of hydrogen peroxide using Vulcan and Printex carbon supports. Carbon, 49(8): 2842–2851
Barazesh J M, Hennebel T, Jasper J T, Sedlak D L (2015). Modular advanced oxidation process enabled by cathodic hydrogen peroxide production. Environmental Science & Technology, 49(12): 7391–7399
Bolton J R, Bircher K G, Tumas W, Tolman C A (2001). Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems-(IUPAC Technical Report). Pure and Applied Chemistry, 73(4): 627–637
Brillas E, Mur E, Casado J (1996). Iron(II) catalysis of the mineralization of aniline using a carbon-PTFE O-2-fed cathode. Journal of the Electrochemical Society, 143(3): L49–L53
Brillas E, Sires I, Oturan M A (2009). Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chemical Reviews, 109(12): 6570–6631
Campos-Martin J M, Blanco-Brieva G, Fierro J L (2006). Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angewandte Chemie International Edition in English, 45(42): 6962–6984
Chaplin B P (2019). The prospect of electrochemical technologies advancing worldwide water treatment. Accounts of Chemical Research, 52(3): 596–604
Chen Z, Chen S, Siahrostami S, Chakthranont P, Hahn C, Nordlund D, Dimosthenis S, Nørskov J K, Bao Z, Jaramillo T F (2017). Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2. Reaction Chemistry & Engineering, 2(2): 239–245
Ciriminna R, Albanese L, Meneguzzo F, Pagliaro M (2016). Hydrogen peroxide: A Key chemical for today’s sustainable development. ChemSusChem, 9(24): 3374–3381
Frangos P, Shen W H, Wang H J, Li X, Yu G, Deng S B, Huang J, Wang B, Wang Y J (2016). Improvement of the degradation of pesticide deethylatrazine by combining UV photolysis with electrochemical generation of hydrogen peroxide. Chemical Engineering Journal, 291: 215–224
Huber M M, Canonica S, Park G Y, Von Gunten U (2003). Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environmental Science & Technology, 37(5): 1016–1024
Lin S, Lu Y, Ye B, Zeng C, Liu G, Li J, Luo H, Zhang R (2020). Pesticide wastewater treatment using the combination of the microbial electrolysis desalination and chemical-production cell and Fenton process. Frontiers of Environmental Science & Engineering, 14(1): 12
Lu S, Wang N, Wang C (2018a). Oxidation and biotoxicity assessment of microcystin-LR using different AOPs based on UV, O3 and H2O2. Frontiers of Environmental Science & Engineering, 12(3): 12
Lu Y B, Liu G L, Luo H P, Zhang R D (2017). Efficient in-situ production of hydrogen peroxide using a novel stacked electrosynthesis reactor. Electrochimica Acta, 248: 29–36
Lu Z, Chen G, Siahrostami S, Chen Z, Liu K, Xie J, Liao L, Wu T, Lin D, Liu Y, Jaramillo T F, Nørskov J K, Cui Y (2018b). High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nature Catalysis, 1(2): 156–162
Oturan M A, Aaron J J, Oturan N, Pinson J (1999). Degradation of chlorophenoxyacid herbicides in aqueous media, using a novel electrochemical method. Pesticide Science, 55(5): 558–562
Paz E C, Aveiro L R, Pinheiro V S, Souza F M, Lima V B, Silva F L, Hammer P, Lanza M R V, Santos M C (2018). Evaluation of H2O2 electrogeneration and decolorization of Orange II azo dye using tungsten oxide nanoparticle-modified carbon. Applied Catalysis B: Environmental, 22: 436–445
Pérez J F, Llanos J, Sáez C, López C, Cañizares P, Rodrigo M A (2019). Towards the scale up of a pressurized-jet microfluidic flow-through reactor for cost-effective electro-generation of H2O2. Journal of Cleaner Production, 211: 1259–1267
Plakas K V, Sklari S D, Yiankakis D A, Sideropoulos G T, Zaspalis V T, Karabelas A J (2016). Removal of organic micropollutants from drinking water by a novel electro-Fenton filter: Pilot-scale studies. Water Research, 91: 183–194
Qiang Z, Chang J H, Huang C P (2002). Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions. Water Research, 36(1): 85–94
Rezaei M, Warsinger D M, Lienhard V J H, Duke M C, Matsuura T, Samhaber W M (2018). Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Research, 139: 329–352
Salmerón I, Plakas K V, Sirés I, Oller I, Maldonado M I, Karabelas A J, Malato S (2018). Optimization of electrocatalytic H2O2 production at pilot plant scale for solar-assisted water treatment. Applied Catalysis B: Environmental, 242: 327–336
Sellers R M (1980). Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) oxalate. Analyst (London), 105(1255): 950–954
Sheng Y P, Zhao Y, Wang X L, Wang R, Tang T (2014). Electrogeneration of H2O2 on a composite acetylene black-PTFE cathode consisting of a sheet active core and a dampproof coating. Electrochimica Acta, 133: 414–421
Stoerzinger K A, Risch M, Han B H, Shao-Horn Y (2015). Recent insights into manganese oxides in catalyzing oxygen reduction kinetics. ACS Catalysis, 5(10): 6021–6031
Tang C, Wang H F, Zhang Q (2018). Multiscale principles to boost reactivity in gas-involving energy electrocatalysis. Accounts of Chemical Research, 51(4): 881–889
Turkay O, Barisci S, Ozturk B, Ozturk H, Dimoglo A (2017a). Electroperoxone treatment of phenol: process comparison, the effect of operational parameters and degradation mechanism. Journal of the Electrochemical Society, 164(9): E180–E186
Turkay O, Ersoy Z G, Barisci S (2017b). Review—the application of an electro-peroxone process in water and wastewater treatment. Journal of the Electrochemical Society, 164(6): E94–E102
USEIA (2016). Annual Electric Sales, Revenue, and Average Price. Washington: U.S. Energy Information Administration
Valim R B, Reis R M, Castro P S, Lima A S, Rocha R S, Bertotti M, Lanza M R V (2013). Electrogeneration of hydrogen peroxide in gas diffusion electrodes modified with tert-butyl-anthraquinone on carbon black support. Carbon, 61: 236–244
von Gunten U (2018). Oxidation processes in water treatment: are we on track? Environmental Science & Technology, 52(9): 5062–5075
von Sonntag C, von Gunten U (2012). Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications. London: IWA Publishing
Wang H, Yuan S, Zhan J, Wang Y, Yu G, Deng S, Huang J, Wang B (2015). Mechanisms of enhanced total organic carbon elimination from oxalic acid solutions by electro-peroxone process. Water Research, 80: 20–29
Wang H, Zhan J, Yao W, Wang B, Deng S, Huang J, Yu G, Wang Y (2018a). Comparison of pharmaceutical abatement in various water matrices by conventional ozonation, peroxone (O3/H2O2), and an electro-peroxone process. Water Research, 130: 127–138
Wang Y, Li X, Zhen L, Zhang H, Zhang Y, Wang C (2012). Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. Journal of Hazardous Materials, 229–230: 115–121
Wang Y, Yu G, Deng S, Huang J, Wang B (2018b). The electro-peroxone process for the abatement of emerging contaminants: Mechanisms, recent advances, and prospects. Chemosphere, 208: 640–654
Wang Y, Zhou W, Gao J, Ding Y, Kou K (2019). Oxidative modification of graphite felts for efficient H2O2 electrogeneration: Enhancement mechanism and long-term stability. Journal of Electroanalytical Chemistry, 833: 258–268
Warsinger D M, Swaminathan J, Guillen-Burrieza E, Arafat H A, Lienhard V J H (2015). Scaling and fouling in membrane distillation for desalination applications: A review. Desalination, 356: 294–313
WHO (2011). Guidelines for Drinking-Water Quality, 4th ed. Geneva: World Health Organization, 324
Xia G, Wang H, Zhan J, Yin X, Wu X, Yu G, Wang Y, Wu M (2020). Evaluation of the stability of polyacrylonitrile-based carbon fiber electrode for hydrogen peroxide production and phenol mineralization during electro-peroxone process. Chemical Engineering Journal, 396: 125291
Xia G, Wang Y, Wang B, Huang J, Deng S, Yu G (2017). The competition between cathodic oxygen and ozone reduction and its role in dictating the reaction mechanisms of an electro-peroxone process. Water Research, 118: 26–38
Yang S, Verdaguer-Casadevall A, Arnarson L, Silvioli L, Čolić V, Frydendal R, Rossmeisl J, Chorkendorff I, Stephens I E L (2018). Toward the decentralized electrochemical production of H2O2: A focus on the catalysis. ACS Catalysis, 8(5): 4064–4081
Yao W, Ur Rehman S W, Wang H, Yang H, Yu G, Wang Y (2018). Pilotscale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Research, 138: 106–117
Yu X M, Zhou M H, Ren G B, Ma L (2015). A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-Fenton. Chemical Engineering Journal, 263: 92–100
Yuan S, Li Z X, Wang Y J (2013). Effective degradation of methylene blue by a novel electrochemically driven process. Electrochemistry Communications, 29: 48–51
Zhang H, Chen S, Zhang H G, Fan X F, Cao C, Yu H T, Quan X (2019). Carbon nanotubes-incorporated MIL-88B-Fe as highly efficient Fenton-like catalyst for degradation of organic pollutants. Frontiers of Environmental Science & Engineering, 13(2): 18
Zhang X K, Zhou Y, Zhao C, Sun Z H, Zhang Z G, Mirza Z A, Saylor G, Zhai J, Zheng H L (2016). Electric field induced activated carbon fiber (ACF) cathode transition from an initiator/a promoter into an electrocatalyst in ozonation process. Chemical Engineering Journal, 304: 129–133
Zhou W, Meng X, Gao J, Alshawabkeh A N (2019). Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review. Chemosphere, 225: 588–607
Acknowledgements
This study is funded by the National Natural Science Foundation of China (Grant No. 51878370), the National Special Program of Water Pollution Control and Management (No. 2017ZX07202), and the special fund of State Key Joint Laboratory of Environment Simulation and Pollution Control (No. 18L01ESPC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Gas diffusion electrode (GDE) is a suitable setup for practical water treatment.
• Electrochemical H2O2 production is an economically competitive technology.
• High current efficiency of H2O2 production was obtained with GDE at 5–400 mA/cm2.
• GDE maintained high stability for H2O2 production for ∼1000 h.
• Electro-generation of H2O2 enhances ibuprofen removal in an E-peroxone process.
Supporting Materials
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, Y., Xia, G. et al. Evaluation of the technoeconomic feasibility of electrochemical hydrogen peroxide production for decentralized water treatment. Front. Environ. Sci. Eng. 15, 1 (2021). https://doi.org/10.1007/s11783-020-1293-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-020-1293-2