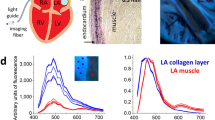
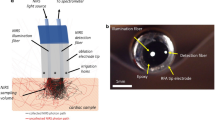
Abstract
Purpose
Multiple studies have shown that spectral analysis of tissue autofluorescence can be used as a live indicator for various pathophysiological states of cardiac tissue, including ischemia, ablation-induced damage, or scar formation. Yet today there are no percutaneous devices that can detect autofluorescence signals from inside a beating heart. Our aim was to develop a prototype catheter to demonstrate the feasibility of doing so.
Methods and Results
Here we summarize technical solutions leading to the development of a percutaneous catheter capable of multispectral imaging of intracardiac surfaces. The process included several iterations of light sources, optical filtering, and image acquisition techniques. The developed system included a compliant balloon, 355 nm laser irradiance, a high-sensitivity CCD, bandpass filtering, and image acquisition synchronized with the cardiac cycle. It enabled us to capture autofluorescence images from multiple spectral bands within the visible range while illuminating the endocardial surface with ultraviolet light. Principal component analysis and other spectral unmixing post-processing algorithms were then used to reveal target tissue.
Conclusion
Based on the success of our prototype system, we are confident that the development of ever more sensitive cameras, recent advances in tunable filters, fiber bundles, and other optical and computational components makes it possible to create percutaneous catheters capable of acquiring hyper or multispectral hypercubes, including those based on autofluorescence, in real-time. This opens the door for widespread use of this methodology for high-resolution intraoperative imaging of internal tissues and organs—including cardiovascular applications.










Similar content being viewed by others
Abbreviations
- AF:
-
Atrial fibrillation
- Auf-HSI:
-
Autofluorescence hyperspectral imaging
- CCD:
-
Charged coupled device
- LA:
-
Left atrium
- LCTF:
-
Liquid crystal tunable filter
- LED:
-
Light emitting diode
- NADH:
-
Nicotinamide adenine dinucleotide
- RF:
-
Radiofrequency
- UV:
-
Ultraviolet Light
References
Ahmad, I., A. Gribble, M. Ikram, M. Pop, and A. Vitkin. Polarimetric assessment of healthy and radiofrequency ablated porcine myocardial tissue. J. Biophotonics 9:750–759, 2016.
Akoum, N., M. Daccarett, C. McGann, N. Segerson, G. Vergara, S. Kuppahally, T. Badger, N. Burgon, T. Haslam, E. Kholmovski, R. Macleod, and N. Marrouche. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 22:16–22, 2011.
Aldhoon, B., T. Kučera, N. Smorodinová, J. Martínek, V. Melenovský, and J. Kautzner. Associations between cardiac fibrosis and permanent atrial fibrillation in advanced heart failure. Physiol. Res. 62:247–255, 2013.
Andreu, D., A. Berruezo, J. T. Ortiz-Pérez, E. Silva, L. Mont, R. Borràs, T. M. de Caralt, R. J. Perea, J. Fernández-Armenta, H. Zeljko, and J. Brugada. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation clinical perspective. Circ. Arrhythm. Electrophysiol. 4:674–683, 2011.
Asfour, H., M. Aljishi, T. Chahbazian, L. M. Swift, N. Muselimyan, D. Gil, and N. A. Sarvazyan. Comparison between autofluorescence and reflectance-based hyperspectral imaging for visualization of atrial ablation lesions. Biophys. J. 110:493a–494a, 2016.
Asfour, H., S. Guan, N. Muselimyan, L. Swift, M. Loew, and N. Sarvazyan. Optimization of wavelength selection for multispectral image acquisition: a case study of atrial ablation lesions. Biomed. Opt. Express 9:2189–2204, 2018.
Bunch, T. J., J. P. Weiss, B. G. Crandall, J. D. Day, J. P. Dimarco, J. D. Ferguson, P. K. Mason, G. McDaniel, J. S. Osborn, D. Wiggins, and S. Mahapatra. Image integration using intracardiac ultrasound and 3D reconstruction for scar mapping and ablation of ventricular tachycardia. J. Cardiovasc. Electrophysiol. 21:678–684, 2010.
Calkins, H., G. Hindricks, R. Cappato, Y. H. Kim, E. B. Saad, L. Aguinaga, J. G. Akar, V. Badhwar, J. Brugada, J. Camm, P. S. Chen, S. A. Chen, M. K. Chung, J. C. Nielsen, A. B. Curtis, D. W. Davies, J. D. Day, A. d’Avila, N. M. S(. Natasja de Groot, L. Di Biase, M. Duytschaever, J. R. Edgerton, K. A. Ellenbogen, P. T. Ellinor, S. Ernst, G. Fenelon, E. P. Gerstenfeld, D. E. Haines, M. Haissaguerre, R. H. Helm, E. Hylek, W. M. Jackman, J. Jalife, J. M. Kalman, J. Kautzner, H. Kottkamp, K. H. Kuck, K. Kumagai, R. Lee, T. Lewalter, B. D. Lindsay, L. Macle, M. Mansour, F. E. Marchlinski, G. F. Michaud, H. Nakagawa, A. Natale, S. Nattel, K. Okumura, D. Packer, E. Pokushalov, M. R. Reynolds, P. Sanders, M. Scanavacca, R. Schilling, C. Tondo, H. M. Tsao, A. Verma, D. J. Wilber, and T. Yamane. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Hear. Rhythm 33(5):369–409, 2017.
Cappato, R., H. Calkins, S.-A. A. Chen, W. Davies, Y. Iesaka, J. Kalman, Y.-H. H. Kim, G. Klein, A. Natale, D. Packer, A. Skanes, F. Ambrogi, and E. Biganzoli. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3:32–38, 2010.
Dana, N., L. Di Biase, A. Natale, S. Emelianov, and R. Bouchard. In vitro photoacoustic visualization of myocardial ablation lesions. Heart Rhythm 11:150–157, 2013.
Deng, H., Y. Bai, A. Shantsila, L. Fauchier, T. S. Potpara, and G. Y. H. Lip. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clin. Res. Cardiol. 106(10):813–823, 2017.
Dooley, K. A., S. Lomax, J. G. Zeibel, C. Miliani, P. Ricciardi, A. Hoenigswald, M. H. Loew, and J. K. Delaney. Mapping of egg yolk and animal skin glue paint binders in Early Renaissance paintings using near infrared reflectance imaging spectroscopy. Analyst 138:4838–4848, 2013.
Dukkipati, S. R., F. Cuoco, I. Kutinsky, A. Aryana, T. D. Bahnson, D. Lakkireddy, I. Woollett, Z. F. Issa, A. Natale, and V. Y. Reddy. Pulmonary vein isolation using the visually guided laser balloon. J. Am. Coll. Cardiol. 66:1350–1360, 2015.
Falco, N. An ICA based approach to hyperspectral image feature reduction. In: Geoscience Remote, 2014.
Fleming, C. P., H. Wang, K. J. Quan, and A. M. Rollins. Real-time monitoring of cardiac radio-frequency ablation lesion formation using an optical coherence tomography forward-imaging catheter. J. Biomed. Opt. 15:030516, 2010.
Gaudi, S., R. Meyer, J. Ranka, J. C. Granahan, S. A. Israel, T. R. Yachik, and D. M. Jukic. Hyperspectral imaging of melanocytic lesions. Am. J. Dermatopathol. 36(2):131–136, 2014.
Germano, G., and D. S. Berman. Clinical Gated Cardiac SPECT. Armonk: Blackwell Futura, 2006.
Gil, D. A., L. M. Swift, H. Asfour, N. Muselimyan, M. A. Mercader, and N. A. Sarvazyan. Autofluorescence hyperspectral imaging of radiofrequency ablation lesions in porcine cardiac tissue. J. Biophotonics 10:1008–1017, 2017.
Guan, S., H. Asfour, N. Sarvazyan, and M. Loew. Application of unsupervised learning to hyperspectral imaging of cardiac ablation lesions. J. Med. Imaging 5:1, 2018.
Guan, S., M. Loew, H. Asfour, N. Sarvazyan, and N. Muselimyan, Lesion detection for cardiac ablation from auto-fluorescence hyperspectral images. In: Progress in Biomedical Optics and Imaging—Proceedings of SPIE, 2018, Vol. 10578.
Holmes, J. W., T. K. Borg, and J. W. Covell. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 7:223–253, 2005.
Iskander-Rizk, S., P. Kruizinga, A. F. W. van der Steen, and G. van Soest. Spectroscopic photoacoustic imaging of radiofrequency ablation in the left atrium. Biomed. Opt. Express 9:1309–1322, 2018.
Kiyotoki, S., J. Nishikawa, T. Okamoto, K. Hamabe, M. Saito, A. Goto, Y. Fujita, Y. Hamamoto, Y. Takeuchi, S. Satori, and I. Sakaida. New method for detection of gastric cancer by hyperspectral imaging: a pilot study. J. Biomed. Opt. 18:26010, 2013.
Lo, L.-W. W., and S.-A. A. Chen. Three-dimensional electroanatomic mapping systems in catheter ablation of atrial fibrillation. Circ. J. 74:18–23, 2010.
Lu, G., and B. Fei. Medical hyperspectral imaging: a review. J. Biomed. Opt. 19:10901, 2014.
Magnani, J. W., M. Rienstra, H. Lin, M. F. Sinner, S. A. Lubitz, D. D. McManus, J. Dupuis, P. T. Ellinor, and E. J. Benjamin. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation 124(18):1982–1993, 2011.
McGann, C. J., E. G. Kholmovski, R. S. Oakes, J. J. E. Blauer, M. Daccarett, N. Segerson, K. J. Airey, N. Akoum, E. Fish, T. J. Badger, E. V. R. DiBella, D. Parker, R. S. MacLeod, and N. F. Marrouche. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J. Am. Coll. Cardiol. 52:1263–1271, 2008.
Muselimyan, N., M. Al Jishi, H. Asfour, L. Swift, and N. A. Sarvazyan. Anatomical and optical properties of atrial tissue: search for a suitable animal model. Cardiovasc. Eng. Technol. 8:505–514, 2017.
Muselimyan, N., L. M. Swift, H. Asfour, T. Chahbazian, R. Mazhari, M. Mercader, and N. A. Sarvazyan. Seeing the invisible: revealing atrial ablation lesions using hyperspectral imaging approach. PLoS ONE 11:e0167760, 2016.
Nazarian, S., and R. Beinart. CMR-guided targeting of gaps after initial pulmonary vein isolation. JACC. Cardiovasc. Imaging 7:664–666, 2014.
Nelson, C., J. McCrohon, F. Khafagi, S. Rose, R. Leano, and T. H. Marwick. Impact of scar thickness on the assessment of viability using dobutamine echocardiography and thallium single-photon emission computed tomography. J. Am. Coll. Cardiol. 43:1248–1256, 2004.
Park, S. Y., R. P. Singh-Moon, E. Y. Wan, and C. P. Hendon. Towards real-time multispectral endoscopic imaging for cardiac lesion quality assessment. Biomed. Opt. Express 10(6):2829–2846, 2019.
Prabhu, S. D., and N. G. Frangogiannis. The biological basis for cardiac repair after myocardial infarction. Circ. Res. 119:91–112, 2016.
Rijnierse, M. T., C. P. Allaart, and P. Knaapen. Principles and techniques of imaging in identifying the substrate of ventricular arrhythmia. J. Nucl. Cardiol. 23:218–234, 2016.
Schade, A., J. Krug, A.-G. Szöllösi, M. El Tarahony, and T. Deneke. Pulmonary vein isolation with a novel endoscopic ablation system using laser energy. Expert Rev. Cardiovasc. Ther. 10:995–1000, 2012.
Shiba, Y., S. Fernandes, W.-Z. Zhu, D. Filice, V. Muskheli, J. Kim, N. J. Palpant, J. Gantz, K. W. Moyes, H. Reinecke, B. Van Biber, T. Dardas, J. L. Mignone, A. Izawa, R. Hanna, M. Viswanathan, J. D. Gold, M. I. Kotlikoff, N. A. Sarvazyan, M. W. Kay, C. E. Murry, and M. A. Laflamme. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489:332–335, 2012.
Singh-Moon, R. P., C. C. Marboe, and C. P. Hendon. Near-infrared spectroscopy integrated catheter for characterization of myocardial tissues: preliminary demonstrations to radiofrequency ablation therapy for atrial fibrillation. Biomed. Opt. Express 6:2494, 2015.
Swift, L. M., H. Asfour, N. Muselimyan, C. Larson, K. Armstrong, and N. A. Sarvazyan. Hyperspectral imaging for label-free in vivo identification of myocardial scars and sites of radiofrequency ablation lesions. Hear. Rhythm 15:564–575, 2018.
Tarabalka, Y., J. A. Benediktsson, and J. Chanussot. Spectral–spatial classification of hyperspectral imagery based on partitional clustering techniques. IEEE Trans. Geosci. Remote Sens. 47:2973–2987, 2009.
Tate, T. H., M. Keenan, J. Black, U. Utzinger, and J. K. Barton. Ultraminiature optical design for multispectral fluorescence imaging endoscopes. J. Biomed. Opt. 22(3):036013, 2017.
Tsutsui, N., M. Yoshida, E. Ito, H. Ohdaira, M. Kitajima, and Y. Suzuki. Laparoscopic cholecystectomy using the PINPOINT® Endoscopic Fluorescence Imaging System with intraoperative fluorescent imaging for acute cholecystitis: a case report. Ann. Med. Surg. 35:146–148, 2018.
Zhao, X., X. Fu, C. Blumenthal, Y. T. Wang, M. W. Jenkins, C. Snyder, M. Arruda, and A. M. Rollins. Integrated RFA/PSOCT catheter for real-time guidance of cardiac radio-frequency ablation. Biomed. Opt. Express 9(12):6400–6411, 2018.
Zhao, X., O. Kilinc, C. J. Blumenthal, D. Dosluoglu, M. W. Jenkins, C. S. Snyder, M. Arruda, and A. M. Rollins. Intracardiac radiofrequency ablation in living swine guided by polarization-sensitive optical coherence tomography. J. Biomed. Opt. 25(5):056001, 2020.
Acknowledgments
We are thankful to our colleagues Drs. Omar Amirana and Narine Muselimyan for useful discussions, expert advice and experimental assistance.
Conflict of interest
Kenneth Armstrong: Employment: Nocturnal Product Development LLC. Funding: HL R41HL12051 & R42HL12051. Stock options: LuxMed Systems. Pending patents: US20150141847A1, US20160120599A1, US201361904018P, US20160143522A1. Granted patents: US9084611B2, US10143517B2. Terrance Ransbury: Employment: LuxMed Systems. Funding: HL R41HL12051 & R42HL12051. Stock options: LuxMed Systems. Pending patents: US20150141847A1, US20160120599A1, US201361904018P, US20160143522A1. Granted patents: US9084611B2, US10143517B2. Cinnamon Larson: Employment: NPD LLC. Funding: HL R41HL12051 & R42HL12051. Stock options: LuxMed Systems. Pending patents: US20150141847A1, US20160120599A1, US201361904018P, US20160143522A1. Granted patents: US9084611B2, US10143517B2. Huda Asfour: Employment: The George Washington University. Funding: HL R41HL12051 & R42HL12051. Narine Sarvazyan: Employment: The George Washington University. Funding: HL R41HL12051 & R42HL12051. Stock options: LuxMed Systems. Pending patents: US20150141847A1, US20160120599A1, US201361904018P. Granted patents: US9014789B2, US9084611B2.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor James E. Moore oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Armstrong, K., Larson, C., Asfour, H. et al. A Percutaneous Catheter for In Vivo Hyperspectral Imaging of Cardiac Tissue: Challenges, Solutions and Future Directions. Cardiovasc Eng Tech 11, 560–575 (2020). https://doi.org/10.1007/s13239-020-00476-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00476-w