Abstract
The recent pandemic situation transpired due to coronavirus novel strain SARS-CoV-2 has become a global concern. This human coronavirus (HCov-19) has put the world on high alert as the numbers of confirmed cases are continuously increasing. The world is now fighting against this deadly virus and is leaving no stone unturned to find effective treatments through testing of various available drugs, including those effective against flu, malaria, etc. With an urgent need for the development of potential strategies, two recent studies from China using Mesenchymal Stem Cells (MSCs) to treat COVID-19 pneumonia have shed some light on a potential cure for the COVID-19 infected patients. However, MSCs, despite being used in various other clinical trials have always been questioned for their tendency to aggregate or form clumps in the injured or disease microenvironment. It has also been reported in various studies that exosomes secreted by these MSCs, contribute towards the cell’s biological and therapeutic efficacy. There have been reports evaluating the safety and feasibility of these exosomes in various lung diseases, thereby proposing them as a cell-free therapeutic agent. Also, attractive features like cell targeting, low-immunogenicity, safety, and high biocompatibility distinguish these exosomes from other synthetic nano-vesicles and thus potentiate their role as a drug delivery nano-platform. Building upon these observations, herein, efforts are made to give an overview of stem cell-derived exosomes as an appealing therapeutic agent and drug delivery nano-carrier. In this review, we briefly recapitulate the recent evidence and developments in understanding exosomes as a promising candidate for novel nano-intervention in the current pandemic scenario. Furthermore, this review will highlight and discuss mechanistic role of exosomes to combat severe lung pathological conditions. We have also attempted to dwell into the nano-formulation of exosomes for its better applicability, storage, and stability thereby conferring them as off the shelf therapeutic.
Similar content being viewed by others
Introduction
In December 2019, cases of pneumonia of unknown causes were reported in Wuhan, Hubei Province, China which was later confirmed to be caused by novel coronavirus SARS-CoV-2 [1]. Later, since its occurrence in the year 2019, it was referred to as COVID-19 [2]. With the outbreak of the COVID-19, it has become a major health concern all over the world as the rate of mortality has been rising each day [3]. At the beginning of the twenty-first century, the world was already introduced to two highly pathogenic CoVs called severe acute respiratory distress syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [4]. These caused global epidemics with alarming morbidity and mortality at a rate of 10% and 34% respectively [5]. COVID-19 is the third type of pathogenic CoV identified in December 2019 in Wuhan, China. The situation is intensifying and the ultimate effect of this outbreak is unclear in the present scenario [6]. Globally, there are over 7,761,609 confirmed cases with about 430,241 deaths as reported by the World Health Organization (WHO) on 15 June 2020. COVID-19 has affected 216 countries and the mortality rate is around 3.4% of the total reported COVID-19 cases worldwide [7]. Currently, there is no proper medication available and the disease poses a new challenge to respiratory medicine.
Patients infected with COVID-19 experience various symptoms, ranging from the mild common cold to severe respiratory illness and death [8, 9]. As per the reports, the virus has an incubation period of ~5 days (range 2–14 days) [10]. The probability of dying, if infected by the virus, differs depending on the age group. Individuals with a previous case history of hypertension, diabetes, respiratory system disease, and cardiovascular disease, are at a higher risk of infection [11]. The vaccine is the most attractive approach to combat SARS viruses and treat infected patients. Scientists and physicians are relentlessly working together worldwide to investigate and decode the exact source of infection, structure, modes of transmission, and immunopathogenic characteristics as well as the most effective treatment strategy [12]. Thus, given the current scenario, where we lack an effective and approved vaccine, and the available potential drugs are still under trials, it is important to look for all possible treatment strategies to cure the infected populations.
COVID-19 Pathogenesis in Lungs
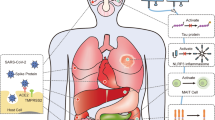
Novel Coronaviruses (nCoVs) are large, enveloped viruses, belonging to the family Coronaviridae in the Nidovirales order [13]. It contains non-segmented positive-sense single-stranded RNA, size ranging from 26 to 32 kb in length. Its structural protein consists of a spike(S), envelope (E), membrane (M), and nucleocapsid (N) protein, which plays a major role in virus entry and replication in the host cell [14]. Various findings have identified COVID-19 to be closely related to two bats derived SARS coronavirus (88% similar), while it was only 70% and 50% related to SARS and MERS-CoV [15]. The structural analysis and pathological insights suggest that the virus enters the lungs and binds to the angiotensin-converting enzyme 2 (ACE-2) receptors which are abundantly expressed on the surface of lungs and intestinal epithelial cells. ACE2 receptors are also expressed by myocardial cells, cholangiocytes, proximal tubular cells of the kidney, etc. [16]. The virus uses the host machinery to replicate itself and infect other cells possessing these receptors. Primarily, these viruses severely affect the lungs causing massive alveolar damage due to imbalanced and excessive immune response which may cause pneumonia and thus, leading to progressive respiratory failure [17]. Some of the major histological changes of the nCOV infected lungs are interstitial inflammation, alveolar damage, intra-alveolar edema, granular tissue formation, fibrin and collagen deposition, bronchiolitis and leukocyte infiltration. These histological changes in the infected patients show clinical manifestations such as fever, cough, fatigue, and shortness of breath and thus need immediate hospitalization [18]. There are still limited data available on nCOV pathogenesis and associated cellular changes, however, understanding the associated changes in the lung is important for diagnosis and management of COVID-19.
Potential Treatment
At present, the treatment is mostly symptomatic and supportive, though anti-inflammatory and antiviral treatment have been employed [19]. Several efforts are underway to make vaccines for this deadly disease, COVID-19, however, it will take approximately 18 months, as estimated by WHO to achieve this goal [20]. Currently, there is no promising and definitive treatment available around the world. The supportive treatment for complicated patients includes invasive mechanical ventilation, continuous renal replacement therapy (CRRT), and even extracorporeal membrane oxygenation (ECMO) [21]. Currently, there are several clinically approved antiviral drugs under consideration to find effective treatments targeting viral entry and replications. These include an oral drug called EIDD-2801, anti-malarial drug hydroxychloroquine, remdesivir -a failed Ebola drug, and kaletra which is a combination of HIV drug lopinavir and ritonavir [22,23,24,25]. Not just anti-viral drugs, but immunosuppressants like sarilumab and tocilizumab are also being tested for the patients, where not only the virus but the patient’s immune overreaction worsens the situation ultimately killing them [26]. Furthermore, dexamethasone which is a generic steroid widely used to reduce inflammation is the latest in the fray and is emerging as another lifesaving drug in this pandemic [27]. Plasma Therapy has also given some hope for severely ill patients where it has been reported to improve their survival rate [28]. Moreover, several studies have shown a shorter hospital stay and lower mortality rates in patients treated with convalescent plasma. Despite these drug modalities under consideration, the question remains the same: Do we have a definitive treatment available for this infection?
Are Mesenchymal Stem Cells Capable of Mitigating the Effect of COVID-19 in Patients, until an Effective Vaccine is Developed?
In the present condition of grim and disparity due to the COVID-19 outbreak, an initial study from China has given us a hope for treating these critically ill patients to combat the virus-induced damages. This study was published in March 2020 and it investigated the potential of MSCs transplantation to improve the outcome of COVID-19 pneumonia patients [29]. It was observed that after a single dose of MSCs intervention, there was a significant improvement in the functional conditions of these patients without any adverse effects. Within 2 days of MSCs transplantation, there was a drastic improvement in the pulmonary function of these patients. Thus, the transplantation of MSCs was found to be effective. Similarly, other clinical trials have been registered for evaluating the efficacy and safety of MSCs isolated from a range of allogenic sources like Bone Marrow, adipose tissue, Wharton’s jelly, and placenta for COVD-19. More than 20 clinical trials are registered tilldate [30].
Such findings using MSCs should not be surprising as they have been well known for their high regenerative and immunomodulatory properties. MSCs can secrete IL-10, hepatocyte growth factor, keratinocytes growth factor, and VEGF to alleviate ARDS, regenerate and repair lung damage and resist fibrosis [31, 32]. MSCs have the potential to inhibit the abnormal activation of T lymphocytes and macrophages, and induce their differentiation into regulatory T cells (Treg) subsets and anti-inflammatory macrophages. These functional aspects of MSCs, make them an ideal candidate amongst other available cellular therapies for the treatment of COVID-19 [30]. Their low immunogenicity due to absence of HLA-Class II antigen, makes them an ideal candidate for allogeneic transplantation. MSCs regenerative and repair mechanisms usually lie in their potential to replace the damaged cells, release anti-inflammatory cytokines, promote angiogenesis, etc. MSCs are the most common andwidely investigated cell type used amongst the other available cell-based therapies in various diseases [33]. Various preclinical studies have already investigated the mechanistic approach of the MSCs to treat respiratory diseases. Based on these studies, the first AETHER trial, published in 2016, evaluated the safety of a single infusion of bone marrow-derived MSCs in nine patients with mild to moderate idiopathic pulmonary fibrosis. No severe adverse events were reported in the next 60 weeks after the infusion of the MSCs. Such studies have formed the base for using MSCs in treating patients with respiratory disorders and have shown to be effective [34].
Despite these clinical reports satisfying the safety and efficacy of using MSCs for respiratory diseases, there are certain challenges and controversies which cannot be neglected at this point considering the COVID-19 situation. Some of these include, high cost of maintenance, long term homing potential, transient survival after transplantation, disturbed differential capacities thus contributing to further adversity of the disease condition, difficult to store and reuse after revival, etc. [35]. Rapid progress in the understanding mechanisms underlying MSCs based cellular therapy has shown profound and significant influence of host inflammatory environment effect on transplanted cells fate. These MSCs are phagocytosed by resident macrophages, and exosomes derived from them can replicate their therapeutic effect. This has paved the way to the paracrine hypothesis which is emerging as one of the most advanced and beneficial therapies over MSCs themselves [36]. The existing literature strongly evidences that the transplanted MSCs when encounter the harsh environment at the target site, they undergo autophagy and apoptosis to release growth factors and cytokine rich exosomes which in turn results in alleviating the disease pathophysiology [37]. Thus, this has brought our attention to new methodologies and approaches concerning these stem cell-derived exosomes as emerging modality, thereby, overcoming the limitations and challenges with that of the parent cells.
Therapeutic Role of MSC Derived Exosomes in Lung Diseases
Exosomes are small nanovesicles of size 30 nm −150 nm secreted by all cell types and known for their role in cell-to-cell communication [38]. These exosomes carry various biologically active molecules like miRNA and proteins. The type of content of these exosomes depends on the parent cell from which they are secreted. These exosomes have cell adhesion molecules present on their surface which help them in binding with the target cell [39]. Since the discovery of exosomes as a mode of the paracrine effect of the MSCs, various studies have investigated their role in deciphering the regenerative and mechanistic aspects in treating various diseases. The use of MSC-derived exosomes as cell-free therapeutics offers several advantages compared to their cellular counterparts such as high stability, low immunogenicity, easy storage, and ability to cross the blood-brain barrier [40]. These exosomes have bilipid membrane composition, potential for off the shelf availability, and biocompatibility that make these exosomes an ideal candidate as a drug delivery vehicle [41]. Thus, these interesting features about exosomes have gained enough interest in evaluating their potential role as a therapeutic and pharmacological intervention in dealing with the present COVID-19 pandemic. Probably, Exosomes therapy can prevent the cytokines stormelicitedby the immune system as the mechanistic approach shown in (Fig. 1; Panel B) and promote endogenous repair by reparative properties of the exosomes. Emerging evidence support the possibility of using MSCs derived exosomes as a new form of therapy for treating different diseases. The initial reports suggesting exosomes as potential therapeutics were in 2009 when identified for their cardio protective role [42]. Later detailed investigations into these MSCs derived exosomes revealed their similar or superior functional capacity like MSCs themselves. A first report in 2012 by Lee et al. demonstrated the cytoprotective effect of exosomes from MSCs in hypoxia-induced pulmonary hypertension mice model [43]. This led to multiple studies investigating exosomes cargo and mechanistic role in various lung diseases including asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), pulmonary arterial hypertension (PAH), and Acute Respiratory distress syndrome (ARDS) [44].
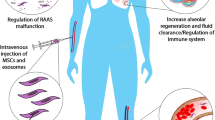
Schematic representation showing the potential role of MSCs derived Exosomes in combating COVID-19 Infection. Panel (a) Synergistic effect of the drug and exosomes may be utilized as an effective approach against the virus. Various hydrophobic and hydrophilic drugs with anti-viral properties can be packaged into exosomes for its delivery to the target site. Panel (b) The therapeutic cargo present in Exosomes aids in the reduction of inflammation, cellular repair, alveolar fluid clearance, and other damage caused to the lung during viral infection
Further, the present pandemic renewed the interest of many researchers towards the applicability of exosomes as an effective and safe therapeutics for combating COVID-19 associated diseases. Recently, bone marrow-derived exosomal based product, EXOFlo was tested in a non-randomized open-label cohort study for the treatment of severe COVID-19. Twenty-four patients were treated with these exosomes and no adverse events were observed within 72 h of treatment. It was observed that exosomes treatment resulted in significant improvement in these patients by restoring oxygen storage capacity, downregulating cytokine storm, and enhancing immunity. However, this study is first of its kind evaluating MSCs derived exosomes therapeutic potential for COVID-19 [45]. Similar studies are warranted to investigate the regenerative and reparative potential of exosomes to further validate the vast pre-clinical observations in non-COVID-19 model systems sharing the same pathologies as that of COVID-19. Based on these studies, the putative role of MSCs derived exosomes in lung pathogenesis can be described as follows:
-
a)
Protection and proliferation of lung epithelial cells: The bronchial and lung epithelial cells represent a primary target during lung infection. During infection, a complex cascade of inflammatory signaling results in the activation of innate and adaptive immune systems against the invading pathogen by these lung epithelial cells [46]. Epithelial cell injury and damage may influence disease risk and thus their protection and proliferation during infection are vital. Different groups have evidence of the potential protective role of these exosomes in various pre-clinical studies. In one study, it was demonstrated that miR-21-5p delivery by the MSC-exosomes protected lung epithelial cells against oxidative stress-induced cell death [47]. In another study, by Bari et al., MSC- exosomes were shown to express Alpha-1-antitrypsin (AAT) on its surface. AAT is a potent inhibitor of neutrophil-derived proteolytic enzymes and has a key role in protecting lung epithelial cells by anti-inflammatory and immunomodulatory effects in the lungs [48]. Kim et al. have also reported that the administration of MSC- exosomes in the animal model of emphysema, increased the proliferation of lung epithelial cells. These exosomes contain growth factor FGF2, which hasthe regenerative capacity and is important in lung development [49].
-
b)
Reversal of Lung inflammation: Cytokines play an important role in immunopathology during viral infection, where the rapid innate immune response is needed as the first line of defense against infection. Deregulated and excessive immune response may be responsible for immune damage to the human body [50, 51]. There is evidence from severely ill patients with CoVs that suggest that proinflammatory response plays a major role in the pathogenesis. In the earlier stages of nCoV infection, respiratory epithelial cells, dendritic cells, and macrophages shows a delayed release of pro-inflammatory cytokines and chemokines [52, 53]. However, in the later stages, these cells secrete low levels of antiviral factors (interferons-IFNs) and high levels of pro-inflammatory cytokines (interleukin -IL-1β, and IL-6) and chemokines (CCl-2, CCL-3, and CCL5). This delayed but elevated levels of pro-inflammatory factors results in cytokine storm which may induce organ damage and therefore, is one of the major concerns contributing to the severity of the disease [54]. These patients are administered immunosuppressive medications, which is inevitably associated with increased risk of infection [55]. MSCs and their derivative exosomes have long been known for their immune-modulatory potential and thus have been evaluated in various pre-clinical and clinical settings. A noteworthy, similar condition is observed in Graft versus host disease (GvHD) associated pulmonary disease [56, 57]. In a preliminary clinical study, MSC- exosomes were shown to alleviate the symptoms of grade IV GvHD patients [58]. Although there is limited data available for exosomes potent immunomodulatory role in the clinical setup. This preliminary study gains confidence in the potential role of exosomes in dealing with the cytokine storm induced lung damage.
-
c)
Polarization of lung macrophages: As previously mentioned, during viral infection, cytokine storm is generated due to the impaired immune response leading to further lung damage. This is largely mediated by pro-inflammatory macrophages in the lungs [59]. The regulation of these pro-inflammatory macrophages (producing inflammatory cytokines such as IL-8, IL-6, IL-1β, and TNF-α) to anti-inflammatory macrophages (producing immunosuppressive cytokines like IL-10 and TGF-β) by exosomes may aid in the further reversal of the disease pathophysiology. Some of the pre-clinical studies evaluating the effect of MSC derived exosomes on lung macrophages in various lung injury models have provided insights into the exosome derived approach as a new strategy for treating nCOV associated pathogenicity. These studies have demonstrated that the presence of several miRNAs like miR-145 and proteins in exosomespromote lung tissue repair and regeneration [60]. Additionally, MSC- exosomes may also modulate phenotype and function of lung-infiltrating dendritic cells (DCs) by inducing the expression of immunosuppressive cytokines like IL-10 and TGF- β and thus preventing the lungs from the detrimental macrophage and DC-driven systemic immune response [61].
-
d)
Reduction in pulmonary edema and lung protein permeability: The disruption of lung endothelial and epithelial barrier during infection causes increased lung protein permeability and alveolar flooding causing pulmonary edema [62]. This in turn disrupts the lung function of air exchange. In a recent study conducted, authors have assessed the effects of systemically administered MSC-exosomes in E. coli endotoxin-induced acute lung injury in mice model. It was found that these exosomes reduced extravascular lung water by 43% with a reduction in pulmonary edema and lung protein permeability [63]. The group later demonstrated the ability of these exosomes in restoring alveolar fluid clearance (AFC) in an ex-vivo lung perfusion model using human donor lungs that were not suitable for transplantation. This ability of MSC-exosomes was in part by a CD-44 dependent mechanism of exosomes for internalization into the damaged host cells [64].
Thus, the administration of MSC- exosomes has high potential to restore the damaged lungs of the patient through various mechanisms, and therefore, could be a possible therapeutic nanomedicine intervention for severely ill patients.
Exosomes as Novel Drug Delivery Nano-Platforms
Exosomes therapeutic efficacy and safety for the delivery of cellular biological components to the target cell has now drawn considerable interest for their potential as drug delivery vehicles. These are emerging as ideal biological nano-carriers for clinical applications owing to their capability of escaping immune recognition and premature degradation, slightly negative zeta potential for longer period of circulation in the body and small size for deeper penetration into the tissues [65]. The idea of using exosomes as a drug delivery system was first reported in 2011 and thereafter has gained increasing attention [66]. Many more studies have been reported, investigating the potential of this biological modality for drug packing and targeted delivery to the sites where cells or synthetic vesicles cannot reach, like a blood-brain barrier. Although, most of the reported studies have evaluated different mechanistic approaches for loading drugs with high efficiency into these vesicles. Two major approaches for loading the therapeutic cargo into the exosome mimetics includes active and passive encapsulation. The passive cargo loading methods are relatively simple and uses the drug loading through diffusion method along the concentration gradient, which further depends on the hydrophobicity of the drug molecule. Different drugs or nano-formulations like curcumin, paclitaxel, etc. have been loaded using this methodology [67]. In contrast, active cargo loading involves methods involving temporary disruption of the exosome-membrane such as sonication, freeze-thaw cycles, electroporation, etc. Various chemotherapeutic drugs, siRNAs, etc. have been loaded into exosomes isolated from various cell sources using this approach [68]. Further modifications in the exosome surface molecules have resulted in enhanced targeted delivery in the damaged tissues [69]. However, there are limited studies available with respect to MSCs (Table 1). Also, combining the therapeutic properties of MSC-exosomes and loading drugs will aid in enhanced regenerative potential (Fig. 1; Panel A). MSCs are known to produce a large number of exosomes, suggesting that these cells may be efficient for exosome production in a clinically applicable scale as compare to other cell sources [65]. As reported in the studies, specifically designed bioreactors can also be utilized for exosome production scale-up [77]. Nevertheless, the idea of using MSC derived exosomes is promising and encouraging as next-generation therapy for combating COVID-19. The regenerative immunomodulatory cargo of these exosomes along with that of anti-viral drugs makes them a novel intervention of treatment and prevention of the disease.
Challenges with Exosomes Therapeutics
Exosomes are gaining attention as a rising star due to their multifaceted role from therapeutics to drug delivery vehicles and diagnostics. However, despite these advantages, there are only a handful of clinical studies available using these nano-vesicles (Table 2). This is due to several challenges associated with exosomes, thereby requiring further investigations [77, 78]. One of the major challenges that need to be addressed is to maintain their stability and functionality over a period of time [79]. Unlike MSCs, exosomes are much more stable and viable at −80 °C for a longer period of time. However, after storage, the freeze-thaw cycles result in the exosome clustering. Furthermore, maintaining low temperature during handling and transportation also causes hindrance in their translational application [80]. Thus, alternative strategies for their storage needs to be evaluated to improve their stability and transportation [81]. To overcome these challenges, several studies have evaluated the applicability of freeze-dried exosomes to preserve them at room temperature. Freeze drying formulations are one of the most reliable methodologies for highly unstable molecules like proteins, nucleic acids, etc. This enhances their practicality by increasing shelf life, lowering storage demands, reducing costs by curtailing cold chain maintenance during transportation [82, 83]. However, clumping of exosomes and degradation of their cargo might be a problem during the freeze-drying process. This can be overcome by adding various stabilizers like sucrose, trehalose, glucose, etc. which aid by replacing the hydration sphere around the exosomes during the freeze-drying process, thus preventing their aggregation and maintaining their membrane integrity [84, 85]. Keeping the aforementioned challenges and applicability of the freeze-dried method in mind, we compared the freeze-dried formulation of exosomes with that of non-freeze-dried formulation. We chose Wharton’s Jelly derived MSCs as a source for exosomes owing to their high immuno-modulatory properties and applicability in COVID-19 like situations [86]. We presented that the freeze-dried formulation maintained the exosome integrity as evident by their size, morphology, and specific surface marker expression (Fig. 2; unpublished data). Furthermore, functional assessment of these exosomes at day7 showed freeze-dried exosomes faring better than the non-freeze-dried exosomes (Fig. 3; unpublished data). Thus, demonstrating the advantage of this methodology in enhancing the availability and applicability of exosomes. These results further validate the fundamental basis for exosome application in biomedical research.
Characterization of exosomes (a-b) The size and morphology of freeze-dried exosomes were similar to non-freeze-dried exosomes. Nanoparticle Tracking Analysis (NTA) showed bell-shaped particle size distribution, peaking at mode 117 nm ±5 nm and 129 nm ±8 nm for freeze-dried exosomes and non-freeze-dried exosomes respectively. There was no aggregation of exosomes observed as indicated by Transmission Electron Microscopy (TEM) (Scale bar-100 nm, Magnification-15000X); (c) Western Blot image showing that freeze-drying method did not alter the exosomal membrane integrity and its protein content as evident by the expression of exosomal specific surface protein CD 63 and cytoplasmic protein Alix; (d) Representative image showing the powdered form of exosomes after freeze-drying
Evaluation of the angiogenic potential of exosomes by in-vitro Matrigel tube formation assay. Exosomes (non- freeze-dried and freeze-dried) stored at various temperatures (RT, 4 °C, −20 °C & −80 °C for 7 days) were evaluated for its tube formation potential at a concentration of 50 μg/ml. (a)Representative Phase-contrast images of the tube formation in each group (Scale bar- 100 μm; Magnification-4X). (b) Quantitative analysis of tube formation for the total number of junctions and total branching length using Image J software *, p < 0.01; **, p < 0.005. Blue dots represent the junctions and red lines represent the branches
Concluding Remarks and Outlook for the Future
The vast advantages of using exosomes over MSCs have shifted the focus on these nano-platforms. The MSCs derived exosomes can be a novel intervention for treating the current COVID-19 pandemic situation due to their regenerative, immunomodulatory, and anti-microbial properties. With upcoming reports and clinical studies using these proposed novel therapeutic interventions, exosomes can be established as cell-free therapeutics and drug delivery modality worldwide. This is the need of the hour and must be looked into as a potential nano-intervention for treating critically ill patients. This review intended to encourage various clinicians/ scientists worldwide working in this area to understand and explore this emerging area of research. Displaying such a diverse range of advantageous properties including their spectral curability, and their capability to be used as a drug carrier, make exosomes an ideal candidate for clinical applications, and as an off the shelf therapeutics.
Even after considering every advancement of utilizing exosomes as a therapeutic aid and a drug delivery vehicle, ultimately the question that still stands is if we are overlooking the efficacy of MSCs derived exosomes in resolving the question of finding a cure to combat COVID-19, or is there no convincing data available in terms of pre-clinical/clinical results to consider the application of these exosomes as a potential treatment for COVID-19 in the current situation? Are we still missing out on the relevant information available with the scientists/ clinicians? There are many such doubts which are hindering the involvement of MSC derived exosomes as a mainstream therapeutic player which may inspire future research in this field. MSCs have been shown to possess the ability to fight against the pathogenesis of COVID-19, yet it is debatable if exosomes derived by them replicate their abilities. Another matter of concern is that despite the similar characterization of MSCs isolated from various tissue sources, there are subtle differences in their response to various disease conditions, so it is unclear whether tissue specificity of MSCs also results in differential modulation in the therapeutic efficacy of the exosomes shed by them. Another application of MSC derived exosomes has been presented in drug delivery, which has made these nano-vesicles an attractive clinical tool. Extensive research is required to modulate their implementation from being mediators of bio-interactions to being drug delivery vehicles. Future, preliminary clinical studies are warranted in this area, as they will also resolve issues inferred by cell-based therapy and put forward an ‘all-in-one’ autonomously therapeutic drug delivery modality.
Abbreviations
- AAT:
-
Alpha-1-antitrypsin
- ACE-2:
-
Angiotensin-converting enzyme 2
- AFC:
-
Alveolar Fluid Clearance
- ARDS:
-
Acute Respiratory Distress Syndrome
- COPD:
-
Chronic Obstructive Pulmonary Disease
- CoV:
-
Coronavirus
- CoV-2:
-
Coronavirus 2002
- COVID-19:
-
Coronavirus 2019
- CRRT:
-
Continuous renal replacement therapy
- ECMO:
-
Even extracorporeal membrane oxygenation
- FGF2:
-
Fibroblast Growth Factor 2
- GvHD:
-
Graft Versus Host Disease
- HCov-19:
-
Human coronavirus 2019
- HIV:
-
Human Immunodeficiency Virus
- IL:
-
Interleukin
- IPF:
-
Idiopathic Pulmonary Fibrosis
- LTB4:
-
Leukotriene B4
- MCP-1:
-
Monocytes chemo-attractants proteins 1
- MERS-CoV:
-
Middle East respiratory syndrome coronavirus
- MRP1:
-
Multi Resistance-Associated Protein 1
- MSCs:
-
Mesenchymal Stem Cells
- PAH:
-
Pulmonary Arterial Hypertension
- RBD:
-
receptor-binding domain
- SARS:
-
Severe Acute Respiratory Distress Syndrome
- TGF-β:
-
Transforming Growth Factor Beta
- VEGF:
-
Vascular Endothelial Growth Factor
- WHO:
-
World Health Organization
References
Du, J., Dong, L., Wang, T., Yuan, C., Fu, R., Zhang, L., Liu, B., Zhang, M., Yin, Y., Qin, J., & Bouey, J. (2020). Psychological symptoms among frontline healthcare workers during COVID-19 outbreak in Wuhan. General Hospital Psychiatry.
Henry, B. M. (2020). COVID-19, ECMO, and lymphopenia: a word of caution. The Lancet Respiratory Medicine, 8(4), e24.
Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). InStatPearls [Internet] 2020. StatPearls Publishing.
Sahin, A. R., Erdogan, A., Agaoglu, P. M., Dineri, Y., Cakirci, A. Y., Senel, M. E., Okyay, R. A., & Tasdogan, A. M. (2020). 2019 novel coronavirus (COVID-19) outbreak: a review of the current literature. EJMO, 4(1), 1–7.
Lee, P. I., & Hsueh, P. R. (2020). Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. Journal of Microbiology, Immunology and Infection.
Heymann, D. L., & Shindo, N. (2020). COVID-19: what is next for public health? The Lancet, 395(10224), 542–545.
Gaye, B., Fanidi, A., & Jouven, X. (2020). Denominator matters in estimating COVID-19 mortality rates. European Heart Journal.
Sun, P., Lu, X., Xu, C., Sun, W., & Pan, B. (2020). Understanding of COVID-19 based on current evidence. Journal of Medical Virology.
Novel Coronavirus (2019-nCoV) Situation Report-7 - World Health Organization (WHO), January 27, 2020.
Coronavirus incubation could be as long as 27 days, Chinese provincial government says - Reuters, Feb. 22, 2020.
Aggarwal, G., Cheruiyot, I., Aggarwal, S., Wong, J., Lippi, G., Lavie, C. J., Henry, B. M., & Sanchis-Gomar, F. (2020). Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: A meta-analysis. Current Problems in Cardiology, 100617.
Sahu, K. K., Lal, A., & Mishra, A. K. (2020). Latest updates on COVID-2019: A changing paradigm shift. Journal of Medical Virology.
Chen, Y., Liu, Q., & Guo, D. (2020). Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology.
Chatterjee S. (2020). Understanding the nature of variations in structural sequences coding for coronavirus spike, envelope, membrane and nucleocapsid proteins of SARS-CoV-2. Envelope, Membrane and Nucleocapsid Proteins of SARS-CoV-2 (March 28, 2020).
Mitra, P., Misra, S., & Sharma, P. (2020). COVID-19 pandemic in India: What lies ahead. Indian Journal of Clinical Biochemistry, 1.
Li, Y., Zhou, W., Yang, L., & You, R. (2020). Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacological Research, 14, 104833.
ul Qamar, M. T., Shahid, F., Ali, U., Fareed, A. Z., & Chen, L. L. (2020). Structural modeling and conserved epitopes prediction against SARS-COV-2 structural proteins for vaccine development. Research Square.
Adhikari, S. P., Meng, S., Wu, Y. J., Mao, Y. P., Ye, R. X., Wang, Q. Z., Sun, C., Sylvia, S., Rozelle, S., Raat, H., & Zhou, H. (2020). Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious Diseases of Poverty, 9(1), 1–12.
Wu, Y. C., Chen, C. S., & Chan, Y. J. (2020). The outbreak of COVID-19: An overview. Journal of the Chinese Medical Association, 83(3), 217.
Hegarty PK, Kamat AM, Zafirakis H, Dinardo A. (2020). BCG vaccination may be protective against Covid-19. preprint.
Wu J, Zha P. (2020). Treatment strategies for reducing damages to lungs in patients with coronavirus and other infections. Available at SSRN 3533279.
Ford, N., Vitoria, M., Rangaraj, A., Norris, S. L., Calmy, A., & Doherty, M. (2020). Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS, or COVID-19: initial assessment. Journal of the International AIDS Society.
Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, Hall MD. Remdesivir: A review of its discovery and development leading to human clinical trials for treatment of COVID–19.
Keshtkar-Jahromi, M., & Bavari, S. (2020). A call for randomized controlled trials to test the efficacy of chloroquine and hydroxychloroquine as therapeutics against novel coronavirus disease (COVID-19). The American Journal of Tropical Medicine and Hygiene, tpmd200230.
Sheahan, T. P., Sims, A. C., Zhou, S., Graham, R. L., Pruijssers, A. J., Agostini, M. L., Leist, S. R., Schäfer, A., Dinnon, K. H., Stevens, L. J., & Chappell, J. D. (2020). An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Science Translational Medicine, 12(541).
Vijayvargiya, P., Garrigos, Z. E., Almeida, N. E., Gurram, P. R., Stevens, R. W., & Razonable, R. R. (2020). Treatment considerations for COVID-19: A critical review of the evidence (or lack thereof). Mayo Clinic Proceedings. Elsevier.
Rosa, S. G., & Santos, W. C. (2020). Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana de Salud Pública, 44, e40.
Chen, L., Xiong, J., Bao, L., & Shi, Y. (2020). Convalescent plasma as a potential therapy for COVID-19. The Lancet Infectious Diseases, 20(4), 398–400.
Leng, Z., Zhu, R., Hou, W., Feng, Y., Yang, Y., Han, Q., Shan, G., Meng, F., Du, D., Wang, S., & Fan, J. (2020). Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease, 11(2), 216–228.
Golchin, A., Seyedjafari, E., & Ardeshirylajimi, A. (2020). Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Reviews and Reports, 1–7.
Cruz, T., & Rojas, M. (2019). Preclinical evidence for the role of stem/stromal cells in targeting ards. InStem Cell-Based Therapy for Lung Disease (pp. 199–217). Cham: Springer.
Zanoni, M., Cortesi, M., Zamagni, A., & Tesei, A. (2019). The role of mesenchymal stem cells in radiation-induced lung fibrosis. International Journal of Molecular Sciences, 20(16), 3876.
Cruz, F. F., & Rocco, P. R. (2020). The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Review of Respiratory Medicine, 14(1), 31–39.
Pelaia, C., Vatrella, A., Sciacqua, A., Terracciano, R., & Pelaia, G. (2020). Role of p38-mitogen-activated protein kinase in COPD: pathobiological implications and therapeutic perspectives. Expert Review of Respiratory Medicine. just-accepted.
Lukomska, B., Stanaszek, L., Zuba-Surma, E., Legosz, P., Sarzynska, S., & Drela, K. (2019). Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells International, 2019.
Gnecchi, M., Danieli, P., Malpasso, G., & Ciuffreda, M. C. (2016). Paracrine mechanisms of mesenchymal stem cells in tissue repair. InMesenchymal stem cells (pp. 123–146). New York: Humana Press.
Hu, C., Zhao, L., Wu, D., & Li, L. (2019). Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia-or ischemia-induced injury. Stem Cell Research & Therapy, 10(1), 120.
Zhou, Y., Kosaka, N., Xiao, Z., & Ochiya, T. (2020). MSC-exosomes in regenerative medicine. InExosomes (pp. 433–465). Academic Press.
Yu, B., Zhang, X., & Li, X. (2014). Exosomes derived from mesenchymal stem cells. International Journal of Molecular Sciences, 15(3), 4142–4157.
Yin, K., Wang, S., & Zhao, R. C. (2019). Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Research, 7(1), 8.
Sarvar, D. P., Shamsasenjan, K., & Akbarzadehlaleh, P. (2016). Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Advanced Pharmaceutical Bulletin, 6(3), 293.
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S., Choo, A., Chen, T. S., Salto-Tellez, M., Timmers, L., Lee, C. N., El Oakley, R. M., & Pasterkamp, G. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4(3), 214–222.
Lee, C., Mitsialis, S. A., Aslam, M., Vitali, S. H., Vergadi, E., Konstantinou, G., Sdrimas, K., Fernandez-Gonzalez, A., & Kourembanas, S. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 126(22), 2601–2611.
Monsel, A., Zhu, Y. G., Gudapati, V., Lim, H., & Lee, J. W. (2016). Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opinion on Biological Therapy, 16(7), 859–871.
Sengupta, V., Sengupta, S., Lazo, A., Woods, P., Nolan, A., & Bremer, N. (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells and Development.
Vareille, M., Kieninger, E., Edwards, M. R., & Regamey, N. (2011). The airway epithelium: soldier in the fight against respiratory viruses. Clinical Microbiology Reviews, 24(1), 210–229.
Wei Li, J., Wei, L., Han, Z., & Chen, Z. (2019). Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. European Journal of Pharmacology, 852, 68–76.
Bari, E., Ferrarotti, I., Di Silvestre, D., Grisoli, P., Barzon, V., Balderacchi, A., Torre, M. L., Rossi, R., Mauri, P., Corsico, A. G., & Perteghella, S. (2019). Adipose mesenchymal extracellular vesicles as Alpha-1-Antitrypsin physiological delivery systems for lung regeneration. Cells, 8(9), 965.
Kim, Y. S., Kim, J. Y., Cho, R., Shin, D. M., Lee, S. W., & Oh, Y. M. (2017). Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Experimental & Molecular Medicine, 49(1), e284.
Walsh, K. B., Teijaro, J. R., Wilker, P. R., Jatzek, A., Fremgen, D. M., Das, S. C., Watanabe, T., Hatta, M., Shinya, K., Suresh, M., & Kawaoka, Y. (2011). Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proceedings of the National Academy of Sciences, 108(29), 12018–12023.
Teijaro, J. R., Walsh, K. B., Cahalan, S., Fremgen, D. M., Roberts, E., Scott, F., Martinborough, E., Peach, R., Oldstone, M. B., & Rosen, H. (2011). Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell, 146(6), 980–991.
Li, G., Fan, Y., Lai, Y., Han, T., Li, Z., Zhou, P., Pan, P., Wang, W., Hu, D., Liu, X., & Zhang, Q. (2020). Coronavirus infections and immune responses. Journal of Medical Virology, 92(4), 424–432.
Pedersen, S. F., & Ho, Y. C. (2020). SARS-CoV-2: a storm is raging. The Journal of Clinical Investigation, 130(5).
Henderson, L. A., Canna, S. W., Schulert, G. S., Volpi, S., Lee, P. Y., Kernan, K. F., Caricchio, R., Mahmud, S., Hazen, M. M., Halyabar, O., & Hoyt, K. J. (2020). On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis & Rheumatology.
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., & Manson, J. J. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet.
Piper C., & Drobyski WR. (2019). Inflammatory cytokine networks in gastrointestinal tract graft vs. host disease. Frontiers in Immunology, 10, 163.
Kamel, A. M., Elsharkawy, N. M., Abdelfattah, E. K., Abdelfattah, R., Samra, M. A., Wallace, P., & Mahmoud, H. K. (2019). IL12 and IFNγ secretion by donor mononuclear cells in response to host antigens may predict acute GVHD after HSCT. Immunobiology, 224(5), 659–665.
Kordelas, L., Rebmann, V., Ludwig, A. K., Radtke, S., Ruesing, J., Doeppner, T. R., Epple, M., Horn, P. A., Beelen, D. W., & Giebel, B. (2014). MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia, 28(4), 970–973.
Kim, J., Yang, Y. L., Jeong, Y., & Jang, Y. S. (2020). Middle East respiratory syndrome-coronavirus infection into established hDDP4-transgenic mice accelerates lung damage via activation of the pro-inflammatory response and pulmonary fibrosis. Journal of Microbiology and Biotechnology, 30, 427–438.
Hao, Q., Gudapati, V., Monsel, A., Park, J. H., Hu, S., Kato, H., Lee, J. H., Zhou, L., He, H., & Lee, J. W. (2019). Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. The Journal of Immunology, 203(7), 1961–1972.
Zeng, S. L., Wang, L. H., Li, P., Wang, W., & Yang, J. (2015). Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Molecular Medicine Reports, 12(2), 2511–2520.
Herrero, R., Sanchez, G., & Lorente, J. A. (2018). New insights into the mechanisms of pulmonary edema in acute lung injury. Annals of Translational Medicine, 6(2).
Zhu, Y. G., Feng, X. M., Abbott, J., Fang, X. H., Hao, Q., Monsel, A., Qu, J. M., Matthay, M. A., & Lee, J. W. (2014). Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells, 32(1), 116–125.
Gennai, S., Monsel, A., Hao, Q., Park, J., Matthay, M. A., & Lee, J. W. (2015). Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. American Journal of Transplantation, 15(9), 2404–2412.
Vader, P., Mol, E. A., Pasterkamp, G., & Schiffelers, R. M. (2016). Extracellular vesicles for drug delivery. Advanced Drug Delivery Reviews, 106, 148–156.
Lakhal, S., & Wood, M. J. (2011). Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays, 33(10), 737–741.
Akuma, P., Okagu, O. D., & Udenigwe, C. C. (2019). Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Frontiers in Sustainable Food Systems, 3, 23.
Liu, C., & Su, C. (2019). Design strategies and application progress of therapeutic exosomes. Theranostics, 9(4), 1015.
Shahabipour, F., Banach, M., & Sahebkar, A. (2016). Exosomes as nanocarriers for siRNA delivery: paradigms and challenges. Archives of Medical Science: AMS, 12(6), 1324.
Aqil, F., Kausar, H., Agrawal, A. K., Jeyabalan, J., Kyakulaga, A. H., Munagala, R., Gupta, R. (2016). Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Experimental and molecular pathology, 101(1), 12–21.
Kim, M. S., Haney, M. J., Zhao, Y., Yuan, D., Deygen, I., Klyachko, N. L., Kabanov, A.V., Batrakova, E. V. (2018). Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine: Nanotechnology, Biology and Medicine, 14(1), 195–204.
Li, Y. J., Wu, J. Y., Wang, J. M., Hu, X. B., Cai, J. X., Xiang, DX. (2020). Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta biomaterialia, 101, 519–30.
Melzer, C., Rehn, V., Yang, Y., Bähre, H., von der Ohe, J., Hass, R. (2019). Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers, 11(6), 798.
Guo, S., Perets, N., Betzer, O., Ben-Shaul, S., Sheinin, A., Michaelevski, I., Popovtzer, R., Offen, D., Levenberg, S. (2019). Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS nano, 13(9), 10015–28.
Chew, J. R., Chuah, S. J., Teo, K. Y., Zhang, S., Lai, R. C., Fu, J. H., Lim, L. P., Lim, S. K., Toh, W. S. (2019). Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta biomaterialia, 89, 252–64.
Cui, G. H., Guo, H. D., Li, H., Zhai, Y., Gong, Z. B., Wu, J., Liu, J. S., Dong, Y. R., Hou, S. X., Liu, J. R. (2019). RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immunity & Ageing, 16(1):10
Cheng, L., Zhang, K., Wu, S., Cui, M., Xu, T. (2017). Focus on mesenchymal stem cell-derived exosomes: opportunities and challenges in cell-free therapy. Stem cells international, 2017.
Yamashita, T., Takahashi, Y., Takakura, Y. (2018). Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biological and Pharmaceutical Bulletin, 41(6), 835–42.
Hood, J. L. (2016). Post isolation modification of exosomes for nanomedicine applications. Nanomedicine, 11(13), 1745–56.
Jeyaram, A., Jay, S. M. (2018). Preservation and storage stability of extracellular vesicles for therapeutic applications. The AAPS journal 20(1), 1.
Ren, J., He, W., Zheng, L., Duan, H. (2016). From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomaterials science, 4(6), 910–21.
Hood, J. L., Scott, M. J., Wickline, S. A. (2014). Maximizing exosome colloidal stability following electroporation. Analytical biochemistry, 448, 41–9.
Fang, X., Duan, Y., Adkins, G. B., Pan, S., Wang, H., Liu, Y., Zhong, W. (2018). Highly Efficient Exosome Isolation and Protein Analysis by an Integrated Nanomaterial-Based Platform. Analytical Chemistry, 90(4), 2787–2795
Bosch, S., de Beaurepaire, L., Allard, M., Mosser, M., Heichette, C., Chrétien, D., Jegou, D., Bach, J. M. (2016). Trehalose prevents aggregation of exosomes and cryodamage. Scientific Reports, 6(1)
Lim, S. K., inventor; Agency for Science Technology, Research, Singapore, assignee. (2019). Method for lyophilising an exosome. United States patent application US 16/340,948.
Wang, Q., Yang, Q., Wang, Z., Tong, H., Ma, L., Zhang, Y., Shan, F., Meng, Y. and Yuan, Z., 2016. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy. Human Vaccines &Immunotherapeutics, 12(1), 85–96.
Acknowledgments
The authors would like to thank Ms. Harshita Sharma, Mr. Vishal, and Mr. Atanu Sen for their technical help during the experiment setup. We also thank Ms. Sharda Ray for her critical evaluation of this manuscript. The work has been supported by the All India Institute of Medical Sciences, New Delhi, and the Department of Biotechnology, Government of India. Graphical abstract was created using BioRender.com.
Author information
Authors and Affiliations
Contributions
P and SG contributed equally in this manuscript conceptualization and writing. VK contributed to graphics, experimentation, and data analysis. YS contributed to writing and critical evaluation of the manuscript. AKD helped in drafting the manuscript. SM analyzed the data, conceived the experiments, and coordinated throughout the manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinky, Gupta, S., Krishnakumar, V. et al. Mesenchymal Stem Cell Derived Exosomes: a Nano Platform for Therapeutics and Drug Delivery in Combating COVID-19. Stem Cell Rev and Rep 17, 33–43 (2021). https://doi.org/10.1007/s12015-020-10002-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10002-z