Abstract
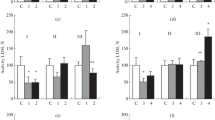
The effect of short-term hypoxia on the activity of oxidoreductases, malate dehydrogenase (MDH, 1.1.1.37) and lactate dehydrogenase (LDH, 1.1.1.27) responsible for urgent adaptation to oxygen deficiency, was studied in the brain and gills (first branchial arch) of the Black Sea scorpionfish Scorpaena porcus. The control group of fish was kept at 4.5–6.7 mg O2 L–1 (normoxia), experimental groups were exposed to 1.7–3.7 mg O2 L–1 (mild hypoxia) and 0.3–1.0 mg O2 L–1 (acute hypoxia); the exposure time was 90 min, water temperature 21–22°C. The dissolved oxygen level was reduced by saturating water with nitrogen. Hypoxia had no significant impact on the brain structures. Under acute hypoxia, MDH and LDH activities, the MDH/LDH index and ATP level in the forebrain, diencephalon and midbrain (FDMB), as well as in the medulla oblongata (MB), remained at the level of control values. Mild hypoxia caused a proportional rise in MDH and LDH activities and an increase in the ATP level in FDMB, most likely, due to a reduced demand for ATP in this brain region. This phenomenon is supposed to be based on the GABAergic mechanism of brain activity regulation, which is able to reduce energy demands of nervous tissue due to increasing the GABAA receptor density. The gills were distinguished by minimum MDH and LDH activities at the background of high MDH/LDH index values. Acute hypoxia led to decrease LDH activity and increase the MDH/LDH index in the gills, reflecting thereby a transition of this organ to an anaerobic operational mode. Under acute hypoxia, there was detected an increase in the relationship within the “MDH activity ↔ LDH activity” system in all types of tissues (r = 0.81–0.94, p < 0.05–0.01), which is typical of the species tolerant to oxygen deficiency. Apparently, this effect is based on MDH coupling with glycolytic substrates under conditions of acute oxygen deficiency, which rules out excessive lactate accumulation under metabolic depression.



Similar content being viewed by others
REFERENCES
Nilsson, G.E. and Renshaw, G.M.C., Hypoxic survival strategies in two fishes: extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shar, J. Exp. Biol., 2004, vol. 207, pp. 3131–3139.
Turesson, J. and Sundin, L., N-methyl-D-aspartate receptors mediate chemoreflex in the shorthorn sculpin Myoxocephalus scorpius, J. Exp. Biol., 2003, vol. 206, pp. 1251–1259.
Taylor, E.W., Leite, C.A.C., McKenzie, D.J., and Wang, T., Control of respiration in fish, amphibians and reptiles, Braz. J. Med. Bio. Res., 2010, vol. 43(5), pp. 409–424.
Saunders, R.L. and Sutterlin, A.M., Cardiac and respiratory responses to hypoxia in the sea raven Hemitripterus americanus and an investigation of possible control mechanisms, J. Fish. Res. Bd. Can., 1971, vol. 28, pp. 491–503.
Sundin, L., Reid, S.G., Kalinin, A.L., Rantin, F.T., and Milsom, W.K., Cardiovascular and respiratory reflexes: the tropical fish, traira (Hoplias malabaricus) O2 chemoresponses, Respir. Physiol., 1999, vol. 116(2–3), pp. 181–199.
Richards, J.G., Metabolic and molecular responses of fish to hypoxia, Fish Physiol., 2009, vol. 27, pp. 443–485.
Nilsson, G.E. and Ostlund-Nilsson, S., Does size matter for hypoxia tolerance in fish? Biol. Rev. Camb. Philos. Soc., 2008, vol. 83(2), pp. 173–189.
Churova, M.V., Meshcheryakova, O.V., and Nemova, N.N., Interrelationship between activities of energy metabolism enzymes and growth rate and size in fish, Uch. Zap. Petrozavod. Gos. Univer., 2011, vol. 4, pp. 31–37.
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation, Princeton, 1984.
Panepucci, R.A., Panepucci, L., Fernandes, M.N., Sanches, J.R., and Rantin, F.T., The effect of hypoxia and recuperation on carbohydrate metabolism in Pacu (Piaractus mesopotamicus), Braz. J. Biol., 2001, vol. 61(4), pp. 547–554.
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation: Mechanism and Process in Physiological Evolution, Oxford, 2002.
Shapiro, A.Z. and Bobkova, A.N., The role of malate dehydrogenase of invertebrates in adaptation to oxygen deficiency, Zh. Evol. Biokhim. Fiziol., 1975, vol. 11, pp. 547–547.
Skorkowski, E.F., Mitochondrial malic enzyme from crustacean and fish muscle, Comp. Biochem. Physiol., 1988, vol. 90(B), pp. 19–24.
Panepucci, L., Fernandes, M.N., Sanches, J.R., and Rantin, F.T., Changes in lactate dehydrogenase and malate dehydrogenase activities during hypoxia and after temperature acclimation in the armored fish, Rhinelepis strigosa (Siluriformes, Loricariidae), Rev. Brasil. Biol., 2000, vol. 60(2), pp. 353–360.
Washizu, T., Nakamura, M., Izawa, N., Suzuki, E., Tsuruno, S., Washizu, M., Nakajo, S., and Arai, T., The activity ratio of the cytosolic MDH/LDH and the isoenzyme pattern of LDH in the peripheral leukocytes of dogs, cats and rabbits, Vet. Res. Commun., 2002, vol. 26(5), pp. 341–346.
Bishop, R.E. and Iliffe, T.M., Ecological physiology of the anchialine shrimp Barbouria cubensis: a comparison of epigean and hypogean populations, Marine Biodiversity, 2012, vol. 42(3), pp. 303–310.
Shul’man, G.E., Stolbov, A.Ya., Soldatov, A.A., Minyuk, G.S., Ivleva, E.V., Trusevich, V.V., and Drobeckaya, I.V., Metabolic reactions of Black Sea fish to long-term experimental hypoxia, Gidrobiol. Zh., 2003, vol. 39(1), pp. 21–30.
Soldatov, A.A., Andreeva, A.Y., Novitskaya, V.N., and Parfenova, I.A., Coupling of membrane and metabolic functions in nucleated erythrocytes of Scorpaena porcus L. under hypoxia in vivo and in vitro, J. Evol. Biochem. Physiol., 2014, vol. 46(4), pp. 409–415.
Lushchak, V.I., Lushchak, L.P., Bahnjukova, T.V., Spichenkov, A.V., and Storey, K.B., Comparative study of free and bound glycolytic enzymes from sea scorpion brain, Biochem. Cell Biol., 1998, vol. 76, pp. 609–614.
Soldatov, A.A., Andreenko, T.I., Golovina, I.V., and Stolbov, A.Y., Peculiarities of organization of tissue metabolism in molluscs with different tolerance to external hypoxia, J. Evol. Biochem. Physiol., 2010, vol. 50(5), pp. 341–349.
Holm-Hansen, O. and Booth, C.R., The measurement of adenosine triphosphate in the Ocean and its ecological significance, Limnol. Oceanogr., 1966, vol. 11(4), pp. 510–519.
Anisimova, I.M. and Lavrovskii, V.V., Ikhtiologiya, Stroenie i nekotorye fiziologicheskie osobennosti ryb, Nervnaya sistema i organy chuvstv (Structure and Some physiological Features of Fish, Nervous System and Sense Organs), Moscow, 1983.
Kotrschal, K., Staaden, M.J., and Huber, R., Fish brains: evolution and environmental relationships, Rev. Fish Biol. Fisheries, 1998, vol. 8(4), pp. 373–408.
Marshal, W.J., Clinical Biochemistry, Elsevier Science, 1995.
Kolesnikova, E.E., Neurophysiological mechanisms of respiratory activity in cyclostomes and fish during aquatic breathing, J. Evol. Biochem. Physiol., 2019, vol. 55(2), pp. 85–96.
Polakof, S., Mommsen, T.P., and Soengas, L.J., Glucosensing and glucose homeostasis: from fish to mammals, Comp. Biochem. Physiol. B, 2011, vol. (160), pp. 123–149.
Nilsson G. E. Evidence for the role of GABA in metabolic depression during anoxia in Crucian carp (Carassius carassius), J. Exp. Biol., 1992, vol. 164, pp. 243–259.
Lutz, P.L. and Leone-Kabler, S.A., Upregulation of GABAA/benzodiazepine receptor during anoxia in the freshwater turtle brain, Am. J. Physiol., 1995, vol. 37, pp. 1332–1335.
Sakurai, S.Y., N-Methyl-D-aspartate binding sites in the vertebrate central nervous system: characterization using quantitative autoradiography of [3H]MK-801 binding, PhD Thesis, University of Michigan, 1992.
Soengas, J.L. and Aldegunde, M., Energy metabolism of fish brain, Comp. Biochem. Physiol. B, Biochem. Molec. Biol., 2002, vol. 131(3), pp. 271–296.
Nilsson, G.E. , Hylland, P., and Löfman, C.O., Anoxia and adenosine induce increased cerebral blood flow rate in crucian carp, Am. J. Physiol., 1994, vol. 267(2), pp. 590–595.
Heath, A.G. and Pritchard, A.W., Effects of severe hypoxia on carbohydrate energy stores and metabolism in two species of fresh-water fish, Physiol. Zool., 1965, vol. 38(4), pp. 325–334.
Silkin, Yu.A. and Silkina, E.N., Effect of hypoxia on physiological-biochemical blood parameters in some marine fish, J. Evol. Biochem. Physiol., 2005, vol. 41(5): 527–532. 2005.
Wilson, D.F., Owen C.S., and Erecinska, M., Quantitative dependence of mitochondrial oxidative phosphorylation on oxygen concentration: a mathematical model, Arch. Biochem. Biophys., 1979, vol. 195(2), pp. 494–504.
Soldatov, A.A., Cytochrome system and oxygen tension in muscle tissue of salt-water fish with different natural activity, J. Evol. Biochem. Physiol., 1996, 32(2), pp. 112–115.
Hochachka, P.W, Buck, L.T., Doll, C.J., and Land, S.C., Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack, Proc. Natl. Acad. Sci. USA, 1996, vol. 93(18), pp. 9493–9498.
Gray, I.E., Comparative study of the gill area of marine fishes, The Biol. Bull., 1954, vol. 107(2), pp. 219–225.
Stammer, A., The microscopic innervation of the gill-apparate in Scoprpaena porcus, Acta Biol., 1966, vol. 12, pp. 101–113.
Payan, P., Girard, J.P., and Mayer-Gostan, N., Branchial ion movements in teleosts: the role of respiratory and chloride cells, Fish Physiol., 1984, vol. 10, pp. 39–63.
Johansen, K., Heart and circulation in gills, skin and lung breathing, Resp. Physiol., 1972, vol. 14, pp. 193–210.
Mommsen, T.P., Metabolism of the fish gill, Fish Physiol., 1984, vol. 10, pp. 203–238.
Sollid, J. and Nilsson, G.E., Plasticity of respiratory structures – adaptive remodeling of fish gills induced by ambient oxygen and temperature, Resp. Physiol. Neurobiol., 2006, vol. 154, pp. 241–251.
Daxboeck, C., Davie, P.S., Perry, S.F., and Randall, D.J., Oxygen uptake in a spontaneously ventilating, blood-perfused trout preparation, J. Exp. Biol., 1982, vol. 101, pp. 35–45.
Cochran, R.E. and Burnett, L.E., Respiratory responses of the salt marsh animals, Fundulus heteroclitus, Leiostomus xanthurus and Palaemonetes pugio, to environmental hypoxia and hypercapnia, J. Exp. Mar. Biol. Ecol., 1996, vol. 195(1), pp. 125–144.
Affonso, E.G., Polez, V.L.P., Correa, C.F., Mazon, A.F., Araujo, M.R.R., Moraes, G., and Rantinc, F.T., Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia, Comp. Biochem. Physiol. C, vol. 133, pp. 375–382.
Wood, C.M., Acid-base and ionic exchanges at gills and kidney after exhaustive exercise in the rainbow trout, J. Exp. Biol., 1988, vol. 136, pp. 461–481.
Cameron, J.N. and Kormanic, G.A., The acid-base responses of gills and kidneys to infused acid and base loads in the Channel catfish, Ictalurus punctatus, J. Exp. Biol., 1982, vol. 99, pp. 143–160.
Holeton, G.F. and Randall, D.J., The effect of hypoxia upon the partial pressure of gases in the blood and water afferent and efferent to the gills of Rainbow trout, J. Exp. Biol., 1967, vol. 46, pp. 317–327.
Randal, D., The control of respiration and circulation in fish during exercise and hypoxia, J. Exp. Biol., 1982, vol. 100, pp. 275–288.
Isaia, J., Girard, J.P., and Payan, P., Kinetic study of gill epithelial permeability to water diffusion in the freshwater trout, Salmo gairdneri: effect of adrenaline, J. Membrane Biol., 1978, vol. 41, pp. 337–347.
Philpott, C.W. and Copeland, D.E., Fine structure of chloride cells from three species of fundulus, J. Cell Biol., 1963, vol. 18(2), pp. 389–404.
Almeida-Val, V.M.F., Farias, I.P., Paula-Silva, M.N., and Duncan, W.P., Biochemical adjustments to hypoxia by amazon cichlids, Braz. J. Med. Biol. Res., 1995, vol. 28, pp. 1257–1263.
Cooper, R.U., Clough, L.M., and Farwell, M.A., West hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus, J. Exp. Mar. Biol. Ecol., 2002, vol. 279(1–2), pp. 1–20.
Funding
This work was implemented within the state assignment to the А.O. Kovalevsky Institute of Biology of the Southern Seas, Russian Academy of Sciences (reg. no. АААА-А18-118021490093-4) and supported in part by the grants from the Government of the Russian Federation (resolution no. 220, agreement no. 14.W03.31.0015) and the Russian Foundation for Basic Research (no. 16-04-00135).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national and institutional principles of handling and using experimental animals for scientific purposes were observed. This study did not involve human subjects as research objects.
Rights and permissions
About this article
Cite this article
Soldatov, A.A., Golovina, I.V., Kolesnikova, E.E. et al. Activity of Energy Metabolism Enzymes and ATP Content in the Brain and Gills of the Black Sea Scorpionfish Scorpaena porcus under Short-Term Hypoxia. J Evol Biochem Phys 56, 224–234 (2020). https://doi.org/10.1134/S0022093020030059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093020030059