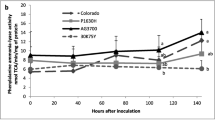
Abstract
Alternative control strategies are increasingly encouraged to develop sustainable crop protection. In this aim, we assessed the ability of Dalgin Active®, an Ascophyllum nodosum extract-based product, to induce resistance in bread wheat and durum wheat against Zymoseptoria tritici, a major fungal pathogen on these crops. Foliar application of the product provided a strong and significant reduction of disease intensity on both wheat species without any direct effect against the pathogen. Infection process monitoring revealed that Dalgin Active® did not prevent fungal epiphytic growth and leaf colonization, but its application results in an inhibition of sporulation as well as fungal cell wall-degrading enzyme and protease activities. During the early stages of infection, Dalgin Active® activated several plant defense markers on both wheat species, including PR protein, antioxidant metabolism, phenylpropanoid, and octadecanoid-based pathways. Although few differences were recorded, the induced defense reaction patterns were overall similar in both wheat species, suggesting that Dalgin Active® could be used to biocontrol Z. tritici on both crops.






Similar content being viewed by others
Change history
02 September 2020
In the original version of this article, Figure 2 is incorrect.
References
Abkhoo J, Sabbagh SK (2016) Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J Appl Phycol 28:1333–1342
Ali N, Farrell A, Ramsubhag A, Jayaraman J (2016a) The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J Appl Phycol 28:1353–1362
Ali N, Ramkissoon A, Ramsubhag A, Jayaraj J (2016b) Ascophyllum extract application causes reduction of disease levels in field tomatoes grown in a tropical environment. Crop Prot 83:67–75
Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, Joubert JM, Pugin A (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant-Microbe Interact 16:1118–1128
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Cheval P, Siah A, Bomble M, Popper AD, Reignault P, Halama P (2017) Evolution of QoI resistance of the wheat pathogen Zymoseptoria tritici in northern France. Crop Prot 92:131–133
Choi HW, Klessig DF (2016) DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol 16:232
Cowger C, Hoffer ME, Mundt CC (2000) Specific adaptation by Mycosphaerella graminicola to a resistant wheat cultivar. Plant Pathol 49:445–451
Douaiher MN, Nowak E, Durand R, Halama P, Reignault P (2007) Correlative analysis of Mycosphaerella graminicola pathogenicity and cell wall degrading enzymes produced in vitro: the importance of xylanases and polygalacturonases. Plant Pathol 56:79–86
El Hadrami I, Baaziz M (1995) Somatic embryogenesis and analysis of peroxidases in Phoenix dactylifera L. Biol Plantarum 37:197–203
Gauthier A, Trouvelot S, Kelloniemi J, Frettinger P, Wendehenne D, Daire X, Joubert JM, Ferrarini A, Delledonne M, Flors V, Poinssot B (2014) The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS One 9:e88145
Hellweg M (2003) Molecular biological and biochemical studies of proteolytic enzymes of the cereal pathogen Fusarium graminearum. PhD Thesis, Bremen 120p
Hernández-Herrera RM, Virgen-Calleros G, Ruiz-López M, Zañudo-Hernández J, Délano-Frier JP, Sánchez-Hernández C (2014) Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani. J Appl Phycol 26:1607–1614
IWGSC, International Wheat Genome Sequencing Consortium (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788
Jannin L, Arkoun M, Etienne P, Laîné P, Goux D, Garnica M, Fuentes M, San Francisco S, Baigorri R, Cruz F, Houdusse F, Garcia-Mina JM, Yvin JC (2013) Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J Plant Growth Regul 32:31–52
Jayaraj J, Wan A, Rahman M, Punja ZK (2008) Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot 27:1360–1366
Jayaraj J, Norrie J, Punja ZK (2011) Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol 23:353–361
Joubert, JM, Yvin JC, Barchietto T, Seng JM, Plesse B, Klarzynski O, Kopp M, Fritig B, Kloareg BA (1998) β 1-3 glucan, specific to a marine alga, stimulates plant defense reactions and induces broad range resistance against pathogens. Brighton Crop Protection Conference: Pests & Diseases; 2: Proceedings of an International Conference, Brighton, UK, 16-19 November 1998. pp 441–448
Kandasamy S, Khan W, Evans F, Critchley AT, Prithiviraj B (2012) Tasco®: a product of Ascophyllum nodosum enhances immune response of Caenorhabditis elegans against Pseudomonas aeruginosa infection. Mar Drugs 10:84–105
Kema GHJ, Yu D, Rijkenberg FHJ, Shaw MW, Baayen R (1996) Histology of the pathogenesis of Mycosphaerella graminicola in wheat. Phytopathology 86:777–786
Keon J, Antoniw J, Carzaniga R, Deller S, Ward JL, Baker JM, Beale MH, Hammond-Kosack K, Rudd JJ (2007) Transcriptional adaptation of Mycosphaerella graminicola to programmed cell death (PCD) of its susceptible wheat host. Mol Plant-Microbe Interact 20:178–193
Klarzynski O, Descamps V, Plesse B, Yvin J-C, Kloareg B, Fritig B (2003) Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol Plant-Microbe Interact 16:115–122
Lovell D, Hunter T, Powers S, Parker SR, Van den Bosch F (2004) Effect of temperature on latent period of septoria leaf blotch on winter wheat under outdoor conditions. Plant Pathol 53:170–181
Nürnberger T, Brunner F, Kemmerling B, Piater L (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198:249–266
Ors ME, Randoux B, Selim S, Siah A, Couleaud G, Maumené C, Sahmer K, Halama P, Reignault P (2018) Cultivar-dependent resistance and associated defense mechanisms in wheat against Zymoseptoria tritici. Plant Pathol 67:561–572
Panjehkeh N, Abkhoo J (2016) Influence of marine brown alga extract (Dalgin) on damping-off tolerance of tomato. J Mater Environ Sci 7:2369–2374
Paulert R, Ebbinghaus D, Urlass C, Moerschbacher BM (2010) Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathol 59:634–642
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:36
Ponomarenko A, Goodwin SB, Kema GH (2011) Septoria tritici blotch (STB) of wheat. Plant Health Instr. https://doi.org/10.1094/PHI-I-2011-0407-01
Randoux B, Renard-Merlier D, Mulard G, Rossard S, Duyme F, Sanssené J, Courtois J, Durand R, Reignault P (2010) Distinct defenses induced in wheat against powdery mildew by acetylated and non acteylated oligogalacturonides. Phytopathology 100:1352–1363
Rayorath HP, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, Alan T, Critchley AT, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.). J Appl Phycol 20:423–429
Renard-Merlier D, Randoux B, Nowak E, Farcy F, Durand R, Reignault P (2007) Iodus 40, salicylic acid, heptanoyl salicylic acid and trehalose exhibit different efficacies and defense targets during a wheat/powdery mildew interaction. Phytochemistry 68:1156–1164
Shukla PS, Mantin EG, Adil M, Bajpai S, Critchley AT, Prithiviraj B (2019) Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance and disease management. Front Plant Sci 10:665
Siah A, Deweer C, Duyme F, Sanssené J, Durand R, Halama P, Reignault P (2010a) Correlation of in planta endo-beta1,4-xylanase activity with the necrotrophic phase of the hemibiotrophic fungus Mycosphaerella graminicola. Plant Pathol 59:661–670
Siah A, Deweer C, Morand E, Reignault P, Halama P (2010b) Azoxystrobin resistance of French Mycosphaerella graminicola strains assessed by four in vitro bioassays and by screening of G143A substitution. Crop Prot 29:737–743
Siah A, Randoux B, Magnin-Robert M, Choma C, Rivière C, Halama P, Reignault P (2018) Natural agents inducing plant resistance against pests and diseases. In: Mérillon JM, Rivière C (eds) Natural antimicrobial agents. Springer, Cham, pp 121–159
Somai-Jemmali L, Randoux B, Siah A, Magnin-Robert M, Halama P, Reignault P, Hamada W (2017a) Similar infection process and induced defense patterns during compatible interactions between Zymoseptoria tritici and both bread and durum wheat species. Eur J Plant Pathol 147:787–801
Somai-Jemmali L, Siah A, Harbaoui K, Ferjaoui S, Halama P, Reignault P, Hamada W (2017b) Correlation between fungal penetration, CWDE activities and defense-related genes with resistance of Tunisian durum wheat cultivars to Zymoseptoria tritici. Physiol Mol Plant Pathol 100:117–125
Stadnik MJ, De Freitas MB (2014) Algal polysaccharides as source of plant resistance inducers. Trop Plant Pathol 39:111–118
Subramanian S, Sangha JS, Gray BA, Singh RP, Hiltz D, Critchley AT, Prithiviraj B (2011) Extracts of the marine brown macroalga, Ascophyllum nodosum, induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Sclerotinia sclerotiorum. Eur J Plant Pathol 131:237–248
Tayeh C, Siah H, Randoux B, Halama P, Walters DR, Reignault P (2014) Topical application of inducers for disease control. In: Walters DR, Newton AC, Lyon GD (eds) Induced resistance for plant defense: a sustainable approach to crop protection. Wiley Blackwell, Oxford, pp 193–230
Tian SM, Weinert J, Zhao QH (2009) Correlation between cell wall-degrading enzymes in wheat leaves infected by Septoria tritici and disease severity. Can J Plant Pathol 31:387–392
Trouvelot S, Varnier AL, Allègre M, Mercier L, Baillieul F, Arnould C, Gianinazzi-Pearson V, Klarzynski O, Joubert JM, Pugin A, Daire X (2008) A beta-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol Plant-Microbe Interact 21:232–243
Walters DR, Bennett AE (2014) Microbial induction of resistance to pathogens. In: Walters DR, Newton AC, Lyon GD (eds) Induced resistance for plant defence: a sustainable approach to crop protection. Blackwell, Oxford, pp 149–170
Acknowledgments
Lamia Somai-Jemmali was supported by Campus France for research internships in Institut Supérieur d’Agriculture - Yncréa Hauts de France (Lille, France) and Université du Littoral Côte d’Opale (Calais, France). This research was carried out in the framework of Alibiotech project founded by the European Union, the French State, and the French Region of Hauts-de-France, and the Bioscreen project (Smartbiocontrol portfolio) founded by the European program Interreg V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the original version of this article, Figure 2 is incorrect.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Somai-Jemmali, L., Siah, A., Randoux, B. et al. Brown alga Ascophyllum nodosum extract-based product, Dalgin Active®, triggers defense mechanisms and confers protection in both bread and durum wheat against Zymoseptoria tritici. J Appl Phycol 32, 3387–3399 (2020). https://doi.org/10.1007/s10811-020-02200-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02200-6